Polypeptides Targeting Paracoccidioides brasiliensis Drk1
- PMID: 37888236
- PMCID: PMC10607314
- DOI: 10.3390/jof9100980
Polypeptides Targeting Paracoccidioides brasiliensis Drk1
Abstract
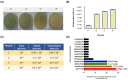
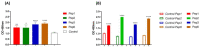
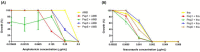
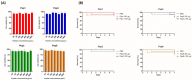
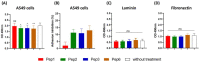
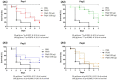
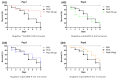
Considering the toxicity of conventional therapeutic approaches and the importance of precise mechanistic targets, it is important to explore signaling pathways implicated in fungal pathobiology. Moreover, treatment of paracoccidioidomycosis, a systemic mycosis caused by a dimorphic fungus, requires prolonged therapeutic regimens. Among the numerous factors underpinning the establishment of Paracoccidioides spp. infection, the capacity to transition from the mycelial to the yeast form is of pivotal importance. The Drk1 protein of Paracoccidioides brasiliensis likely plays a decisive role in this morphological shift and subsequent virulence. We identified peptides with affinity for the PbDrk1 protein using the phage-display method and assessed the effects of these peptides on P. brasiliensis. The peptides were found to inhibit the phase transition of P. brasiliensis. Furthermore, a substantial proportion of these peptides prevented adhesion to pneumocytes. Although these peptides may not possess inherent antifungal properties, they can augment the effects of certain antifungal agents. Notably, the cell wall architecture of P. brasiliensis appears to be modulated by peptide intervention, resulting in a reduced abundance of glycosylated proteins and lipids. These peptides were also evaluated for their efficacy in a Galleria mellonella model and shown to contribute to enhanced larval survival rates. The role of PbDrk1, which is notably absent in mammals, should be further investigated to improve the understanding of its functional role in P. brasiliensis, which may be helpful for designing novel therapeutic modalities.
Keywords: Drk1; Paracoccidioides brasiliensis; peptides; phage-display.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
- 2016/17048-4/São Paulo Research Foundation
- 164217/2020-7/National Council for Scientific and Technological Development
- 88881.141306/2017-01/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- x/Programa de Apoio ao Desenvolvimento Científico da Faculdade de Ciências Farmacêuticas da UNESP PADC-FCF-UNESP
LinkOut - more resources
Full Text Sources
Miscellaneous

