Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis
- PMID: 37888404
- PMCID: PMC10606145
- DOI: 10.3390/gels9100832
Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis
Abstract
Background: Dermatitis is skin disorder that is complicated by recurrent infections of skin by bacteria, viruses, and fungi. Spilanthol is an active constituent of Spilanthes acmella, which possess strong anti-bacterial properties. The purpose of this study was to develop a herbal emulgel for the treatment of dermal bacterial infections, as microscopic organisms have created solid resistance against anti-microbials.
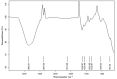
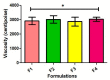
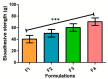
Methods: Emulgels were prepared and characterized for parameters such as physical examination, rheological studies, spreading coefficient, bio-adhesive strength measurement, extrudability study, antibacterial activity, FTIR analysis, in vitro drug dissolution, and ex vivo permeation studies.
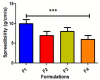
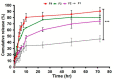
Result: With a statistically significant p-value = 0.024, 100% antibacterial activity was observed by F4 against Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli (mean ± S.D) (25.33 ± 0.28, 27.33 ± 0.5, and 27 ± 0.5). However, maximum antibacterial effect 100% formulations produced zones of inhibitions against E. colip-value = 0.001. The mean zone of inhibition produced by F4 was greatest among all at 26.44 ± 0.37 mm (mean ± S.D). The F4 formulation produced a maximum percentage dissolution, permeation, and flux of 86.35 ± 0.576, 55.29 ± 0.127%, and 0.5532 ug/cm2/min, respectively.
Conclusions: The present study therefore, suggests the use of S. acmella extract and olive oil containing emulgel for treating bacterial skin infections.
Keywords: Escherichia coli; Pseudomonas aeruginosa; Spilanthes acmella; Staphylococcus aureus; emulgel; spilanthol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Simpson E.L., Silverberg J.I., Nosbaum A., Winthrop K.L., Guttman-Yassky E., Hoffmeister K.M., Egeberg A., Valdez H., Zhang M., Farooqui S.A. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am. J. Clin. Dermatol. 2021;22:693–707. doi: 10.1007/s40257-021-00618-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

