Crotoxin Modulates Macrophage Phenotypic Reprogramming
- PMID: 37888647
- PMCID: PMC10611389
- DOI: 10.3390/toxins15100616
Crotoxin Modulates Macrophage Phenotypic Reprogramming
Abstract
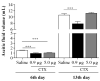
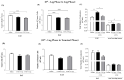
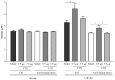
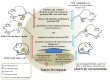
Macrophage plasticity is a fundamental feature of the immune response since it favors the rapid and adequate change of the functional phenotype in response to the pathogen or the microenvironment. Several studies have shown that Crotoxin (CTX), the major toxin of the Crotalus durissus terrificus snake venom, has a long-lasting antitumor effect both in experimental models and in clinical trials. In this study, we show the CTX effect on the phenotypic reprogramming of macrophages in the mesenchymal tumor microenvironment or those obtained from the peritoneal cavity of healthy animals. CTX (0.9 or 5 μg/animal subcutaneously) administered concomitantly with intraperitoneal inoculation of tumor cells (1 × 107/0.5 mL, injected intraperitoneally) of Ehrlich Ascitic Tumor (EAT) modulated the macrophages phenotype (M1), accompanied by increased NO• production by cells from ascites, and was evaluated after 13 days. On the other hand, in healthy animals, the phenotypic profile of macrophages was modulated in a dose-dependent way at 0.9 μg/animal: M1 and at 5.0 μg/animal: M2; this was accompanied by increased NO• production by peritoneal macrophages only for the dose of 0.9 μg/animal of CTX. This study shows that a single administration of CTX interferes with the phenotypic reprogramming of macrophages, as well as with the secretory state of cells from ascites, influencing events involved with mesenchymal tumor progression. These findings may favor the selection of new therapeutic targets to correct compromised immunity in different systems.
Keywords: cytokines; immunomodulatory effect; macrophage plasticity; rattlesnake; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Solinas G., Schiarea S., Liguori M., Fabbri M., Pesce S., Zammataro L., Pasqualini F., Nebuloni M., Chiabrando C., Mantovani A., et al. Tumor-Conditioned Macrophages Secrete Migration-Stimulating Factor: A New Marker for M2-Polarization, Influencing Tumor Cell Motility. J. Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

