Branched-Selective Cross-Electrophile Coupling of 2-Alkyl Aziridines and (Hetero)aryl Iodides Using Ti/Ni Catalysis
- PMID: 37888947
- PMCID: PMC10704858
- DOI: 10.1021/jacs.3c08301
Branched-Selective Cross-Electrophile Coupling of 2-Alkyl Aziridines and (Hetero)aryl Iodides Using Ti/Ni Catalysis
Abstract
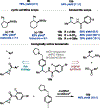
The arylation of 2-alkyl aziridines by nucleophilic ring-opening or transition-metal-catalyzed cross-coupling enables facile access to biologically relevant β-phenethylamine derivatives. However, both approaches largely favor C-C bond formation at the less-substituted carbon of the aziridine, thus enabling access to only linear products. Consequently, despite the attractive bond disconnection that it poses, the synthesis of branched arylated products from 2-alkyl aziridines has remained inaccessible. Herein, we address this long-standing challenge and report the first branched-selective cross-coupling of 2-alkyl aziridines with aryl iodides. This unique selectivity is enabled by a Ti/Ni dual-catalytic system. We demonstrate the robustness of the method by a twofold approach: an additive screening campaign to probe functional group tolerance and a feature-driven substrate scope to study the effect of the local steric and electronic profile of each coupling partner on reactivity. Furthermore, the diversity of this feature-driven substrate scope enabled the generation of predictive reactivity models that guided mechanistic understanding. Mechanistic studies demonstrated that the branched selectivity arises from a TiIII-induced radical ring-opening of the aziridine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







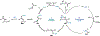
Similar articles
-
Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides.J Am Chem Soc. 2020 Apr 22;142(16):7598-7605. doi: 10.1021/jacs.0c01724. Epub 2020 Apr 6. J Am Chem Soc. 2020. PMID: 32250602 Free PMC article.
-
Nickel-Catalyzed Enantioselective C(sp3)-C(sp3) Cross-Electrophile Coupling of N-Sulfonyl Styrenyl Aziridines with Alkyl Bromides.J Am Chem Soc. 2024 Sep 18;146(37):25426-25432. doi: 10.1021/jacs.4c08435. Epub 2024 Sep 4. J Am Chem Soc. 2024. PMID: 39231321
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Nucleophilic ring opening reactions of aziridines.Mol Divers. 2018 May;22(2):447-501. doi: 10.1007/s11030-018-9829-0. Epub 2018 May 4. Mol Divers. 2018. PMID: 29728870 Review.
-
Iodoarene Activation: Take a Leap Forward toward Green and Sustainable Transformations.Chem Rev. 2025 Mar 26;125(6):3440-3550. doi: 10.1021/acs.chemrev.4c00808. Epub 2025 Mar 7. Chem Rev. 2025. PMID: 40053418 Free PMC article. Review.
Cited by
-
Selective C-N Bond Cleavage in Unstrained Pyrrolidines Enabled by Lewis Acid and Photoredox Catalysis.J Am Chem Soc. 2024 Nov 6;146(44):30698-30707. doi: 10.1021/jacs.4c13210. Epub 2024 Oct 23. J Am Chem Soc. 2024. PMID: 39440606 Free PMC article.
-
Manganese-mediated C(sp)-Si cross-electrophile coupling of alkynyl halides with chlorosilanes.Chem Sci. 2025 Jul 3;16(31):14216-14224. doi: 10.1039/d5sc03449c. eCollection 2025 Aug 6. Chem Sci. 2025. PMID: 40636578 Free PMC article.
-
Green Light Promoted Iridium(III)/Copper(I)-Catalyzed Addition of Alkynes to Aziridinoquinoxalines Through the Intermediacy of Azomethine Ylides.Angew Chem Int Ed Engl. 2024 Mar 18;63(12):e202318876. doi: 10.1002/anie.202318876. Epub 2024 Feb 12. Angew Chem Int Ed Engl. 2024. PMID: 38267370 Free PMC article.
-
β-Phenethylamine Synthesis: N-Pyridinium Aziridines as Latent Dual Electrophiles.Angew Chem Int Ed Engl. 2024 Jul 29;63(31):e202406335. doi: 10.1002/anie.202406335. Epub 2024 Jun 14. Angew Chem Int Ed Engl. 2024. PMID: 38699820 Free PMC article.
References
-
- Liu M; Kong D; Li M; Zi G; Hou G Iridium-Catalyzed Enantioselective Hydrogenation of β,Β-Disubstituted Nitroalkenes. Adv. Synth. Catal. 2015, 357 (18), 3875–3879. 10.1002/adsc.201500723. - DOI

