The three-dimensional structure of the EBV genome plays a crucial role in regulating viral gene expression in EBVaGC
- PMID: 37889078
- PMCID: PMC10711448
- DOI: 10.1093/nar/gkad936
The three-dimensional structure of the EBV genome plays a crucial role in regulating viral gene expression in EBVaGC
Abstract
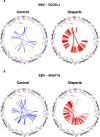
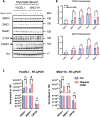
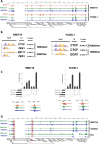
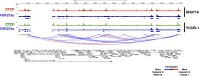
Epstein-Barr virus (EBV) establishes lifelong asymptomatic infection by replication of its chromatinized episomes with the host genome. EBV exhibits different latency-associated transcriptional repertoires, each with distinct three-dimensional structures. CTCF, Cohesin and PARP1 are involved in maintaining viral latency and establishing episome architecture. Epstein-Barr virus-associated gastric cancer (EBVaGC) represents 1.3-30.9% of all gastric cancers globally. EBV-positive gastric cancers exhibit an intermediate viral transcription profile known as 'Latency II', expressing specific viral genes and noncoding RNAs. In this study, we investigated the impact of PARP1 inhibition on CTCF/Cohesin binding in Type II latency. We observed destabilization of the binding of both factors, leading to a disrupted three-dimensional architecture of the episomes and an altered viral gene expression. Despite sharing the same CTCF binding profile, Type I, II and III latencies exhibit different 3D structures that correlate with variations in viral gene expression. Additionally, our analysis of H3K27ac-enriched interactions revealed differences between Type II latency episomes and a link to cellular transformation through docking of the EBV genome at specific sites of the Human genome, thus promoting oncogene expression. Overall, this work provides insights into the role of PARP1 in maintaining active latency and novel mechanisms of EBV-induced cellular transformation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

