Convalescent Plasma for Covid-19-Induced ARDS in Mechanically Ventilated Patients
- PMID: 37889107
- PMCID: PMC10755833
- DOI: 10.1056/NEJMoa2209502
Convalescent Plasma for Covid-19-Induced ARDS in Mechanically Ventilated Patients
Abstract
Background: Passive immunization with plasma collected from convalescent patients has been regularly used to treat coronavirus disease 2019 (Covid-19). Minimal data are available regarding the use of convalescent plasma in patients with Covid-19-induced acute respiratory distress syndrome (ARDS).
Methods: In this open-label trial, we randomly assigned adult patients with Covid-19-induced ARDS who had been receiving invasive mechanical ventilation for less than 5 days in a 1:1 ratio to receive either convalescent plasma with a neutralizing antibody titer of at least 1:320 or standard care alone. Randomization was stratified according to the time from tracheal intubation to inclusion. The primary outcome was death by day 28.
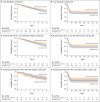
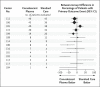
Results: A total of 475 patients underwent randomization from September 2020 through March 2022. Overall, 237 patients were assigned to receive convalescent plasma and 238 to receive standard care. Owing to a shortage of convalescent plasma, a neutralizing antibody titer of 1:160 was administered to 17.7% of the patients in the convalescent-plasma group. Glucocorticoids were administered to 466 patients (98.1%). At day 28, mortality was 35.4% in the convalescent-plasma group and 45.0% in the standard-care group (P = 0.03). In a prespecified analysis, this effect was observed mainly in patients who underwent randomization 48 hours or less after the initiation of invasive mechanical ventilation. Serious adverse events did not differ substantially between the two groups.
Conclusions: The administration of plasma collected from convalescent donors with a neutralizing antibody titer of at least 1:160 to patients with Covid-19-induced ARDS within 5 days after the initiation of invasive mechanical ventilation significantly reduced mortality at day 28. This effect was mainly observed in patients who underwent randomization 48 hours or less after ventilation initiation. (Funded by the Belgian Health Care Knowledge Center; ClinicalTrials.gov number, NCT04558476.).
Copyright © 2023 Massachusetts Medical Society.
Figures
Comment in
-
Convalescent Plasma for Covid-19-Induced ARDS.N Engl J Med. 2024 Jan 25;390(4):384-385. doi: 10.1056/NEJMc2313675. N Engl J Med. 2024. PMID: 38265659 No abstract available.
-
Convalescent Plasma for Covid-19-Induced ARDS.N Engl J Med. 2024 Jan 25;390(4):385. doi: 10.1056/NEJMc2313675. N Engl J Med. 2024. PMID: 38265660 No abstract available.
-
Convalescent Plasma for Covid-19-Induced ARDS.N Engl J Med. 2024 Jan 25;390(4):385-386. doi: 10.1056/NEJMc2313675. N Engl J Med. 2024. PMID: 38265661 No abstract available.
-
Convalescent Plasma for Covid-19-Induced ARDS. Reply.N Engl J Med. 2024 Jan 25;390(4):386. doi: 10.1056/NEJMc2313675. N Engl J Med. 2024. PMID: 38265662 No abstract available.
-
Convalescent plasma: An unexpected new therapeutic option for critically ill COVID-19 patients coming from the past.J Clin Anesth. 2024 Jun;94:111411. doi: 10.1016/j.jclinane.2024.111411. Epub 2024 Feb 8. J Clin Anesth. 2024. PMID: 38335905 No abstract available.
References
-
- Johns Hopkins Coronavirus Resource Center. COVID-19 dashboard (https://coronavirus.jhu.edu/map.html).
-
- ICNARC. Reports: COVID-19 report. July 2021. (https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports).
