Human Plaque Myofibroblasts to Study Mechanisms of Atherosclerosis
- PMID: 37889192
- PMCID: PMC10727388
- DOI: 10.1161/JAHA.123.030243
Human Plaque Myofibroblasts to Study Mechanisms of Atherosclerosis
Abstract
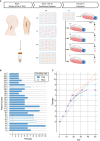
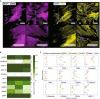
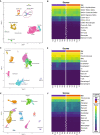
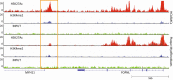
Background Plaque myofibroblasts are critical players in the initiation and advancement of atherosclerotic disease. They are involved in the production of extracellular matrix, the formation of the fibrous cap, and the underlying lipidic core via modulation processes in response to different environmental cues. Despite clear phenotypic differences between myofibroblast cells and healthy vascular smooth muscle cells, smooth muscle cells are still widely used as a cellular model in atherosclerotic research. Methods and Results Here, we present a conditioned outgrowth method to isolate and culture myofibroblast cells from plaques. We obtained these cells from 27 donors (24 carotid and 3 femoral endarterectomies). We show that they keep their proliferative capacity for 8 passages, are transcriptionally stable, retain donor-specific gene expression programs, and express extracellular matrix proteins (FN1, COL1A1, and DCN) and smooth muscle cell markers (ACTA2, MYH11, and CNN1). Single-cell transcriptomics reveals that the cells in culture closely resemble the plaque myofibroblasts. Chromatin immunoprecipitation sequencing shows the presence of histone H3 lysine 4 dimethylation at the MYH11 promoter, pointing to their smooth muscle cell origin. Finally, we demonstrated that plaque myofibroblasts can be efficiently transduced (>97%) and are capable of taking up oxidized low-density lipoprotein and undergoing calcification. Conclusions In conclusion, we present a method to isolate and culture cells that retain plaque myofibroblast phenotypical and functional capabilities, making them a suitable in vitro model for studying selected mechanisms of atherosclerosis.
Keywords: disease modeling; myofibroblast; phenotypic modulation; plaque cells; smooth muscle cell.
Figures



References
-
- Martos‐Rodríguez CJ, Albarrán‐Juárez J, Morales‐Cano D, Caballero A, MacGrogan D, de la Pompa JL, Carramolino L, Bentzon JF. Fibrous caps in atherosclerosis form by Notch‐dependent mechanisms common to arterial media development. Arterioscler Thromb Vasc Biol. 2021;41:e427–e439. doi: 10.1161/ATVBAHA.120.315627 - DOI - PubMed
-
- Newman AAC, Serbulea V, Baylis RA, Shankman LS, Bradley X, Alencar GF, Owsiany K, Deaton RA, Karnewar S, Shamsuzzaman S, et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat Metab. 2021;3:166–181. doi: 10.1038/s42255-020-00338-8 - DOI - PMC - PubMed
-
- Hartman RJG, Owsiany K, Ma L, Koplev S, Hao K, Slenders L, Civelek M, Mokry M, Kovacic JC, Pasterkamp G, et al. Sex‐stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation. 2021;143:713–726. doi: 10.1161/CIRCULATIONAHA.120.051231 - DOI - PMC - PubMed
-
- Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single‐cell analysis. Nat Med. 2019;25:1280–1289. doi: 10.1038/s41591-019-0512-5 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

