Prognostic value of chronic kidney disease in patients undergoing left atrial appendage occlusion
- PMID: 37889200
- PMCID: PMC10653166
- DOI: 10.1093/europace/euad315
Prognostic value of chronic kidney disease in patients undergoing left atrial appendage occlusion
Abstract
Aims: Atrial fibrillation (AF) and chronic kidney disease (CKD) often coexist and share an increased risk of thrombo-embolism (TE). CKD concomitantly predisposes towards a pro-haemorrhagic state. Our aim was to evaluate the prognostic value of CKD in patients undergoing percutaneous left atrial appendage occlusion (LAAO).
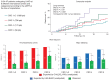
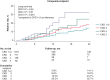
Methods and results: A total of 2124 consecutive AF patients undergoing LAAO were categorized into CKD stage 1+2 (n = 1089), CKD stage 3 (n = 796), CKD stage 4 (n = 170), and CKD stage 5 (n = 69) based on the estimated glomerular filtration rate at baseline. The primary endpoint included cardiovascular (CV) mortality, TE, and major bleeding. The expected annual TE and major bleeding risks were estimated based on the CHA2DS2-VASc and HAS-BLED scores. A non-significant higher incidence of major peri-procedural adverse events (1.7 vs. 2.3 vs. 4.1 vs. 4.3) was observed with worsening CKD (P = 0.14). The mean follow-up period was 13 ± 7 months (2226 patient-years). In comparison to CKD stage 1+2 as a reference, the incidence of the primary endpoint was significantly higher in CKD stage 3 (log-rank P-value = 0.04), CKD stage 4 (log-rank P-value = 0.01), and CKD stage 5 (log-rank P-value = 0.001). Left atrial appendage occlusion led to a TE risk reduction (RR) of 72, 66, 62, and 41% in each group. The relative RR of major bleeding was 58, 44, 51, and 52%, respectively.
Conclusion: Patients with moderate-to-severe CKD had a higher incidence of the primary composite endpoint. The relative RR in the incidence of TE and major bleeding was consistent across CKD groups.
Keywords: Atrial fibrillation; Chronic kidney disease; Left atrial appendage; Stroke; Watchman.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: G.B.C. received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Boston Scientific, and Acutus Medical. C.dA. received research grants on behalf of the centre from Biotronik, Medtronic, Abbott, LivaNova, Boston Scientific, AtriCure, Philips, and Acutus and compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Livanova, Boston Scientific, Atricure, Acutus Medical, and Daiichi Sankyo. J.C.G. is a consultant and has received speaker fees from Boston Scientific, Abbott, Medtronic, Biotronik, Pfizer, Bayer, Novartis, Daiichi Sankyo, and Boehringer Ingelheim. M.M. serves as a consultant for Boston Scientific and Abbott. L.D.B. is a consultant for Stereotaxis, Biosense Webster, Boston Scientific, Rhythm Management, and Abbott Medical. J.S. is a consultant for Boston Scientific, Abbott, Baylis, Gore, and FEops, and a proctor for Boston Scientific and Abbott. D.G. is a consultant for Boston Scientific, Abbot, Acutus, and Biosense Webster. A.N. is as a consultant for Abbott, Biosense Webster, Inc., Biotronik, Boston Scientific, Baylis, and Medtronic. The remaining authors have nothing to disclose.
Figures

Comment in
-
Left atrial appendage occlusion in chronic kidney disease: opening the way to randomized trials.Europace. 2023 Nov 2;25(11):euad342. doi: 10.1093/europace/euad342. Europace. 2023. PMID: 37963108 Free PMC article. No abstract available.
References
-
- Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. - PubMed
-
- Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. - PubMed
-
- Kar S, Doshi SK, Sadhu A, Horton R, Osorio J, Ellis C, et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: results from the PINNACLE FLX trial. Circulation 2021;143:1754–62. - PubMed
-
- Gianni C, Horton RP, Della Rocca DG, Mohanty S, Al-Ahmad A, Bassiouny MA, et al. Intracardiac echocardiography – versus transesophageal echocardiography – guided left atrial appendage occlusion with watchman FLX. J Cardiovasc Electrophysiol 2021;32:2781–4. - PubMed
-
- Della Rocca DG, Magnocavallo M, Gianni C, Mohanty S, Natale VN, Al-Ahmad A, et al. Procedural and short-term follow-up outcomes of Amplatzer Amulet occluder versus Watchman FLX device: a meta-analysis. Heart Rhythm 2022;19:1017–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

