The efficacy and safety of cannabidiol as adjunct treatment for drug-resistant idiopathic epilepsy in 51 dogs: A double-blinded crossover study
- PMID: 37889215
- PMCID: PMC10658598
- DOI: 10.1111/jvim.16912
The efficacy and safety of cannabidiol as adjunct treatment for drug-resistant idiopathic epilepsy in 51 dogs: A double-blinded crossover study
Abstract
Background: Approximately 30% of dogs with idiopathic epilepsy (IE) are drug-resistant. Recent studies have suggested cannabidiol (CBD) may be an effective anticonvulsant in dogs with IE.
Objective: To evaluate the addition of CBD to antiseizure drugs (ASDs) on seizure frequency and to report adverse events in dogs with drug-resistant IE.
Animals: Fifty-one dogs. Dogs having at least 2 seizures per month while receiving at least 1 ASD were included in the trial.
Methods: Double-blinded placebo-controlled crossover study. The 5 mg/kg/day dosage met futility requirements after 12 dogs, and a dosage of 9 mg/kg/day was used in the next 39 dogs. Dogs were randomly assigned to receive CBD or placebo for 3 months, with a 1-month washout period between oils. Total numbers of seizures and seizure days were recorded. Diagnostic testing was performed periodically throughout the trial.
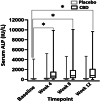
Results: At the 9 mg/kg/day dose, the decrease in total seizure frequency was significant compared with placebo. A 24.1% decrease in seizure days occurred in dogs receiving CBD and a 5.8% increase occurred in dogs receiving placebo (P ≤ .05). No significant difference was found in the number of responders (≥50% decrease in total seizures or seizure days). Liver enzyme activities increased at both dosages. Decreased appetite and vomiting were more common in the CBD phase (P ≤ .05).
Conclusions and clinical importance: Cannabidiol decreased total seizures and seizure days compared to placebo when administered to dogs PO at 9 mg/kg/day. Liver enzymes should be monitored with administration of CBD in dogs.
Keywords: CBD; canine; cannabis; epileptic; seizures.
© 2023 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



References
-
- Monteiro R, Adams V, Keys D, Platt SR. Canine idiopathic epilepsy: prevalence, risk factors and outcome associated with cluster seizures and status epilepticus. J Small Anim Pract. 2012. Sep;53(9):526‐530. - PubMed
-
- Berendt M, Gredal H, Ersbøll AK, Alving J. Premature death, risk factors, and life patterns in dogs with epilepsy. J Vet Intern Med. 2007;21(4):754‐759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

