Safety and pharmacokinetics of BI 685509, a soluble guanylyl cyclase activator, in patients with cirrhosis: A randomized Phase Ib study
- PMID: 37889522
- PMCID: PMC10615399
- DOI: 10.1097/HC9.0000000000000276
Safety and pharmacokinetics of BI 685509, a soluble guanylyl cyclase activator, in patients with cirrhosis: A randomized Phase Ib study
Abstract
Background: Portal hypertension is a severe complication of cirrhosis. This Phase Ib study (NCT03842761) assessed the safety, tolerability, and pharmacokinetics of soluble guanylyl cyclase activator BI 685509 in patients with mild or moderate hepatic impairment (Child-Pugh [CP] A or B cirrhosis) and healthy volunteers (HVs).
Methods: In this single-center, randomized, placebo-controlled study, patients received BI 685509 (maximum doses: 1, 2, or 3 mg, twice daily [BID]) or placebo for 28 days. HVs received one 0.5 mg dose of BI 685509 or placebo.
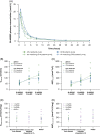
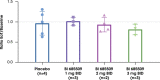
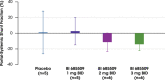
Results: In total, 64 participants (CP-A, n=24; CP-B, n=25; HVs, n=15) were included; most commonly with NAFLD (36.7%), alcohol-associated (30.6%), or chronic viral hepatitis-related cirrhosis (28.6%). In patients with CP-A cirrhosis, drug-related adverse events (AEs) occurred in 5.6% of BI 685509-treated patients and 16.7% of placebo recipients. In patients with CP-B cirrhosis, drug-related AEs occurred in 26.3% of BI 685509-treated patients only. No serious AEs occurred in patients with CP-A cirrhosis; in patients with CP-B cirrhosis, serious AEs (not drug-related) occurred in 10.5% of BI 685509-treated patients and 16.7% of patients receiving placebo. BI 685509 was rapidly absorbed; exposure increased with dosage and was similar between etiologies and between patients with CP-A cirrhosis and patients with CP-A cirrhosis but lower in HVs. The mean percentage portal-systemic shunt fraction was measured in patients with CP-A cirrhosis and decreased at the end of treatment in the 2 mg BID (-11.2 ± 11.9%) and 3 mg BID (-14.0 ± 8.4%) BI 685509 dose groups, but not in the placebo group (+1.0 ± 27.3%).
Conclusion: BI 685509 was generally well tolerated, with 3 serious, not drug-related AEs reported in patients with CP-B cirrhosis. In patients with CP-A cirrhosis, portal-systemic shunt fraction in the exploratory efficacy analysis was reduced by 2 mg BID and 3 mg BID BI 685509.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Eric J. Lawitz received grants from 89bio, Allergan, Akero Therapeutics, AstraZeneca, Axcella Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, DURECT, Eli Lilly, Elobix, Enanta, ENYO, Galmed, GENFIT, Gilead, Hanmi, Intercept, Laboratory for Advanced Medicine, Madrigal, Merck, Metacrine, Novartis, Novo Nordisk, Octeta, Poxel, Roche, Viking, and Zydus. Thomas Reiberger consults, advises, is on the speakers' bureau, received grants, and received compensation from AbbVie and Gilead. He consults, advises, received grants, and received compensation from Boehringer Ingelheim. He consults, advises, is on the speakers' bureau, and received grants from Intercept and MSD. He consults, advises, and received grants from Siemens. He consults and advises Bayer. He is on the speakers' bureau and received grants from Gore. He is on the speakers' bureau and received compensation from Roche. He received grants from MYR, Philips Healthcare, and Pliant. Jörn M. Schattenberg consults and received grants from Boehringer Ingelheim, Gilead, and Siemens Healthcare. He consults for Bayer, Bristol Myers Squibb, GENFIT, Intercept, Madrigal, Merck, Nordic Bioscience, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi. He is on the speakers' bureau for Falk Foundation. Corinna Schoelch is employed by Boehringer Ingelheim. Harvey O. Coxson is employed by Boehringer Ingelheim. Diane Wong is employed by Boehringer Ingelheim. Judith Ertle is employed by Boehringer Ingelheim.
Figures


References
-
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2017;65:310–335. - PubMed
-
- Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. . Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–2261. - PubMed
-
- Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. . Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

