Pain and itch coding mechanisms of polymodal sensory neurons
- PMID: 37889748
- PMCID: PMC10729537
- DOI: 10.1016/j.celrep.2023.113316
Pain and itch coding mechanisms of polymodal sensory neurons
Abstract
Pain and itch coding mechanisms in polymodal sensory neurons remain elusive. MrgprD+ neurons represent a major polymodal population and mediate both mechanical pain and nonhistaminergic itch. Here, we show that chemogenetic activation of MrgprD+ neurons elicited both pain- and itch-related behavior in a dose-dependent manner, revealing an unanticipated compatibility between pain and itch in polymodal neurons. While VGlut2-dependent glutamate release is required for both pain and itch transmission from MrgprD+ neurons, the neuropeptide neuromedin B (NMB) is selectively required for itch signaling. Electrophysiological recordings further demonstrated that glutamate synergizes with NMB to excite NMB-sensitive postsynaptic neurons. Ablation of these spinal neurons selectively abolished itch signals from MrgprD+ neurons, without affecting pain signals, suggesting a dedicated itch-processing central circuit. These findings reveal distinct neurotransmitters and neural circuit requirements for pain and itch signaling from MrgprD+ polymodal sensory neurons, providing new insights on coding and processing of pain and itch.
Keywords: CP: Neuroscience; MrgprD; glutamate; itch; neural coding; neuromedin B; neurotransmitters; pain; polymodal sensory neurons.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
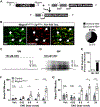
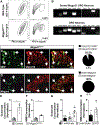

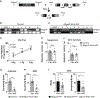


References
-
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, and McKemy DD (2013). A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J. Neurosci. 33, 2837–2848. 10.1523/JNEUROSCI.1943-12.2013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

