Spatiotemporal genomic profiling of intestinal metaplasia reveals clonal dynamics of gastric cancer progression
- PMID: 37890493
- PMCID: PMC10729843
- DOI: 10.1016/j.ccell.2023.10.004
Spatiotemporal genomic profiling of intestinal metaplasia reveals clonal dynamics of gastric cancer progression
Abstract
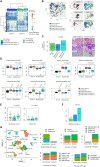
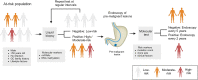
Intestinal metaplasia (IM) is a pre-malignant condition of the gastric mucosa associated with increased gastric cancer (GC) risk. Analyzing 1,256 gastric samples (1,152 IMs) across 692 subjects from a prospective 10-year study, we identify 26 IM driver genes in diverse pathways including chromatin regulation (ARID1A) and intestinal homeostasis (SOX9). Single-cell and spatial profiles highlight changes in tissue ecology and IM lineage heterogeneity, including an intestinal stem-cell dominant cellular compartment linked to early malignancy. Expanded transcriptome profiling reveals expression-based molecular subtypes of IM associated with incomplete histology, antral/intestinal cell types, ARID1A mutations, inflammation, and microbial communities normally associated with the healthy oral tract. We demonstrate that combined clinical-genomic models outperform clinical-only models in predicting IMs likely to transform to GC. By highlighting strategies for accurately identifying IM patients at high GC risk and a role for microbial dysbiosis in IM progression, our results raise opportunities for GC precision prevention and interception.
Keywords: cancer screening; cell-of-origin; gastric cancer; intestinal metaplasia; pre-cancer; single-cell sequencing; spatial transcriptomics; targeted DNA sequencing; transcriptome sequencing.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.T. has stock in Tempus Healthcare, previous funding from Kyowa Hakko Kirin and Thermo Fisher Scientific, and patents/other intellectual property through the Agency for Science and Technology Research, Singapore (all outside the submitted work). K.G.Y. is a co-inventor on patents “Serum MicroRNA Biomarker for the Diagnosis of Gastric Cancer” and “Methods Related to Real-Time Cancer Diagnostics at Endoscopy Utilizing Fiber-Optic Raman Spectroscopy”; a member of Scientific Advisory Board of MiRXES Pte Ltd. He has no stock or shares in the related companies. He has no conflicts of interest to disclose regarding this submitted work. R.S. has received honoraria from MSD, Eli Lilly, BMS, Roche, Taiho, Astra Zeneca, DKSH, and Ipsen; has advisory activity with Bristol Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD, GSK, DKSH, and Astellas; received research funding from Paxman Coolers, MSD, and Natera; and has received travel grants from Roche, Astra Zeneca, Taiho, Eisai, and DKSH.
Figures







Comment in
-
From intestinal metaplasia to gastric cancer: Witnessing the rise of evil over time and space.Cancer Cell. 2023 Dec 11;41(12):2011-2013. doi: 10.1016/j.ccell.2023.10.005. Epub 2023 Oct 26. Cancer Cell. 2023. PMID: 37890491
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Leung W.K., Wu M.S., Kakugawa Y., Kim J.J., Yeoh K.G., Goh K.L., Wu K.C., Wu D.C., Sollano J., Kachintorn U., et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. - PubMed
-
- Dan Y.Y., So J.B.Y., Yeoh K.G. Endoscopic screening for gastric cancer. Clin. Gastroenterol. Hepatol. 2006;4:709–716. - PubMed
-
- Hsu M., Safadi A.O., Lui F. StatPearls; 2022. Physiology, Stomach. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

