A conserved core region of the scaffold NEMO is essential for signal-induced conformational change and liquid-liquid phase separation
- PMID: 37890781
- PMCID: PMC10694592
- DOI: 10.1016/j.jbc.2023.105396
A conserved core region of the scaffold NEMO is essential for signal-induced conformational change and liquid-liquid phase separation
Abstract
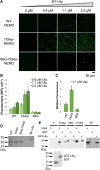
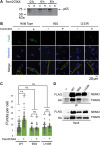
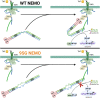
Scaffold proteins help mediate interactions between protein partners, often to optimize intracellular signaling. Herein, we use comparative, biochemical, biophysical, molecular, and cellular approaches to investigate how the scaffold protein NEMO contributes to signaling in the NF-κB pathway. Comparison of NEMO and the related protein optineurin from a variety of evolutionarily distant organisms revealed that a central region of NEMO, called the Intervening Domain (IVD), is conserved between NEMO and optineurin. Previous studies have shown that this central core region of the IVD is required for cytokine-induced activation of IκB kinase (IKK). We show that the analogous region of optineurin can functionally replace the core region of the NEMO IVD. We also show that an intact IVD is required for the formation of disulfide-bonded dimers of NEMO. Moreover, inactivating mutations in this core region abrogate the ability of NEMO to form ubiquitin-induced liquid-liquid phase separation droplets in vitro and signal-induced puncta in vivo. Thermal and chemical denaturation studies of truncated NEMO variants indicate that the IVD, while not intrinsically destabilizing, can reduce the stability of surrounding regions of NEMO due to the conflicting structural demands imparted on this region by flanking upstream and downstream domains. This conformational strain in the IVD mediates allosteric communication between the N- and C-terminal regions of NEMO. Overall, these results support a model in which the IVD of NEMO participates in signal-induced activation of the IKK/NF-κB pathway by acting as a mediator of conformational changes in NEMO.
Keywords: IKK; NEMO; NF-kappaB; conformational change; liquid-liquid phase separation; mutant; optineurin; scaffold protein; signal transduction.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




Update of
-
A Conserved Core Region of the Scaffold NEMO is Essential for Signal-induced Conformational Change and Liquid-liquid Phase Separation.bioRxiv [Preprint]. 2023 May 25:2023.05.25.542299. doi: 10.1101/2023.05.25.542299. bioRxiv. 2023. Update in: J Biol Chem. 2023 Dec;299(12):105396. doi: 10.1016/j.jbc.2023.105396. PMID: 37292615 Free PMC article. Updated. Preprint.
References
-
- Ghosh S. CRC Press; Boca Rotan, FL: 2006. Handbook of NF-kappaB; p. 232.
-
- Trares K., Ackermann J., Koch I. The canonical and non-canonical NF-κB pathways and their crosstalk: a comparative study based on petri nets. Biosystems. 2022;211 - PubMed
-
- Maubach G., Schmädicke A.-C., Naumann M. NEMO links nuclear factor-κB to human diseases. Trends Mol. Med. 2017;23:1138–1155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

