Associations of health-related quality of life with major adverse cardiovascular and cerebrovascular events for individuals with ischaemic heart disease: systematic review, meta-analysis and evidence mapping
- PMID: 37890894
- PMCID: PMC10619110
- DOI: 10.1136/openhrt-2023-002452
Associations of health-related quality of life with major adverse cardiovascular and cerebrovascular events for individuals with ischaemic heart disease: systematic review, meta-analysis and evidence mapping
Erratum in
-
Correction: Associations of health-related quality of life with major adverse cardiovascular and cerebrovascular events for individuals with ischaemic heart disease: systematic review, meta-analysis and evidence mapping.Open Heart. 2023 Nov 27;10(2):e002452corr1. doi: 10.1136/openhrt-2023-002452corr1. Open Heart. 2023. PMID: 38011996 Free PMC article. No abstract available.
Abstract
Objective: To investigate the association between health-related quality of life (HRQoL) and major adverse cardiovascular and cerebrovascular events (MACCE) in individuals with ischaemic heart disease (IHD).
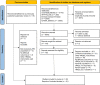
Methods: Medline(R), Embase, APA PsycINFO and CINAHL (EBSCO) from inception to 3 April 2023 were searched. Studies reporting association of HRQoL, using a generic or cardiac-specific tool, with MACCE or components of MACCE for individuals with IHD were eligible for inclusion. Risk of bias was assessed using the Newcastle-Ottawa Quality Assessment Scale to assess the quality of the studies. Descriptive synthesis, evidence mapping and random-effects meta-analysis were performed stratified by HRQoL measures and effect estimates. Between-study heterogeneity was assessed using the Higgins I2 statistic.
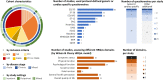
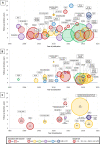
Results: Fifty-one articles were included with a total of 134 740 participants from 53 countries. Meta-analysis of 23 studies found that the risk of MACCE increased with lower baseline HeartQoL score (HR 1.49, 95% CI 1.16 to 1.93) and Short Form Survey (SF-12) physical component score (PCS) (HR 1.39, 95% CI 1.28 to 1.51). Risk of all-cause mortality increased with a lower HeartQoL (HR 1.64, 95% CI 1.34 to 2.01), EuroQol 5-dimension (HR 1.17, 95% CI 1.12 to 1.22), SF-36 PCS (HR 1.29, 95% CI 1.19 to 1.41), SF-36 mental component score (HR 1.18, 95% CI 1.08 to 1.30).
Conclusions: This study found an inverse association between baseline values or change in HRQoL and MACCE or components of MACCE in individuals with IHD, albeit with between-study heterogeneity. Standardisation and routine assessment of HRQoL in clinical practice may help risk stratify individuals with IHD for tailored interventions.
Prospero registration number: CRD42021234638.
Keywords: cardiovascular outcomes; health-related quality of life; meta-analysis; mortality; systematic review.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare the following: AS has received grant from the European Society of Cardiology, support from AstraZeneca, Bayer, Novartis, Gedeon Richter, Amgen and Servier—outside the submitted work; CPG has received grants or contracts from Alan Turing Institute, British Heart Foundation, National Institute for Health Research, Horizon 2020, Abbott Diabetes, Bristol Myers Squibb, European Society of Cardiology; consulting fees from AI Nexus, AstraZeneca, Amgen, Bayer, Bristol Myers Squibb, Boehrinher-Ingleheim, CardioMatics, Chiesi, Daiichi Sankyo, GPRI Research, Menarini, Novartis, iRhyth, Organon, The Phoenix Group, Payment or honoraria from AstraZeneca, Boston Scientific, Menarini, Novartis, Raisio Group, Wondr Medical, Zydus, support form AstraZeneca, participation on a Data Safety Monitoring Board or Advisory Board in DANBLCOK trial and TARGET CTCA trial, leadership or fiduciary role in EHJ Quality of Care and Clinical Outcomes as Deputy Editor, NICE Indicator Advisory Committee, Chair ESC Quality Indicator Committee, Stock or stock options CardioMatics Receipt of Kosmos device. SA has received support for attending meetings and/or travel from the European Society of Cardiology and participates on a Data Safety Monitoring Board or Advisory Board of EuroHeart; HVS has received grants from CIHR and Heart and Stroke Foundation; GB has received fees to the institution from Bayer and Pfizer, honoraria for lectures from AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Novo Nordisk, Pfizer and Sanofi—outside of present work. Other authors have nothing to declare.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
