The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases
- PMID: 37891183
- PMCID: PMC10611796
- DOI: 10.1038/s41467-023-42305-3
The α-synuclein PET tracer [18F] ACI-12589 distinguishes multiple system atrophy from other neurodegenerative diseases
Abstract
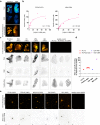
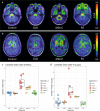
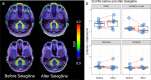
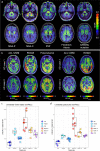
A positron emission tomography (PET) tracer detecting α-synuclein pathology will improve the diagnosis, and ultimately the treatment of α-synuclein-related diseases. Here we show that the PET ligand, [18F]ACI-12589, displays good in vitro affinity and specificity for pathological α-synuclein in tissues from patients with different α-synuclein-related disorders including Parkinson's disease (PD) and Multiple-System Atrophy (MSA) using autoradiography and radiobinding techniques. In the initial clinical evaluation we include 23 participants with α-synuclein related disorders, 11 with other neurodegenerative disorders and eight controls. In vivo [18F]ACI-12589 demonstrates clear binding in the cerebellar white matter and middle cerebellar peduncles of MSA patients, regions known to be highly affected by α-synuclein pathology, but shows limited binding in PD. The binding statistically separates MSA patients from healthy controls and subjects with other neurodegenerative disorders, including other synucleinopathies. Our results indicate that α-synuclein pathology in MSA can be identified using [18F]ACI-12589 PET imaging, potentially improving the diagnostic work-up of MSA and allowing for detection of drug target engagement in vivo of novel α-synuclein targeting therapies.
© 2023. Springer Nature Limited.
Conflict of interest statement
R.S. has received a speaker fee from Roche. F.C., E.V., J.M., T.T., V.H., I.K.D., R.L.-C., A. Pfeifer, M.K.-V., and J.S. are employees of AC Immune SA. M.S. is an employee of Antaros Medical. A Puschmann receives reimbursement from Elsevier for his work as Associate Editor for Parkinsonism and Related Disorders. P.S. has received consultancy/speaker fees from Abbvie, Amylyx, Lundbeck, and Takeda. C.S. and D.S.R. are employees of Invicro, LLC. O.H. has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Amylyx, Alzpath, BioArctic, Biogen, Cerveau, Eisai, Eli Lilly, Fujirebio, Genentech, Merck, Novartis, Novo Nordisk, Roche, Sanofi, and Siemens. T.O., J.J., M.A., P.M.-A., C.H., E.S., S.H., K.B., and E.T.L. report no disclosures.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

