Kinetics of adaptive immune responses after administering mRNA-Based COVID-19 vaccination in individuals with and without prior SARS-CoV-2 infections
- PMID: 37891503
- PMCID: PMC10604405
- DOI: 10.1186/s12879-023-08728-5
Kinetics of adaptive immune responses after administering mRNA-Based COVID-19 vaccination in individuals with and without prior SARS-CoV-2 infections
Abstract
Objective: We aimed to compare the adaptive immune response in individuals with or without prior SARS-CoV-2 infections following the administration of mRNA-based COVID-19 vaccines.
Methods: A total of 54 participants with ages ranging from 37 to 56 years old, consisting of 23 individuals without a history of SARS-CoV-2 infection (uninfected group) and 31 individuals with prior infection of SARS-CoV-2 (infected group) who have received two doses of mRNA SARS-CoV-2 vaccines were enrolled in this study. We measured the IFN-γ level upon administration of BNT162b2 (PF) or mRNA-1273 (MO) by QuantiFERON SARS-CoV-2. The production of neutralizing antibodies was evaluated by a surrogate virus neutralization assay, and the neutralizing capacity was assessed by a plaque reduction neutralization test (PRNT50). The immune response was compared between the two groups.
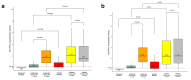
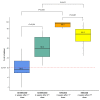
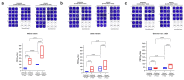
Results: A significantly higher level of IFN-γ (p < 0.001) and neutralization antibodies (p < 0.001) were observed in the infected group than those in the uninfected group following the first administration of vaccines. The infected group demonstrated a significantly higher PRNT50 titer than the uninfected group against the Wuhan strain (p < 0.0001). Still, the two groups were not significantly different against Delta (p = 0.07) and Omicron (p = 0.14) variants. Following the second vaccine dose, T- and B-cell levels were not significantly increased in the infected group.
Conclusion: A single dose of mRNA-based COVID-19 vaccines would boost immune responses in individuals who had previously contracted SARS-CoV-2.
Keywords: COVID-19; IFN-γ; Immune response; Neutralizing antibody; mRNA vaccine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Evaluation of the T cell and B cell response following the administration of COVID-19 vaccines in Korea.J Microbiol Immunol Infect. 2022 Dec;55(6 Pt 1):1013-1024. doi: 10.1016/j.jmii.2022.09.004. Epub 2022 Sep 28. J Microbiol Immunol Infect. 2022. PMID: 36261313 Free PMC article.
-
Safety and immunogenicity against ancestral, Delta and Omicron virus variants following a booster dose of an inactivated whole-virus COVID-19 vaccine (VLA2001): Interim analysis of an open-label extension of the randomized, controlled, phase 3 COV-COMPARE trial.J Infect. 2023 Sep;87(3):242-254. doi: 10.1016/j.jinf.2023.06.022. Epub 2023 Jul 3. J Infect. 2023. PMID: 37406777 Clinical Trial.
-
The temporal course of T- and B-cell responses to vaccination with BNT162b2 and mRNA-1273.Clin Microbiol Infect. 2022 May;28(5):701-709. doi: 10.1016/j.cmi.2021.09.006. Epub 2021 Sep 20. Clin Microbiol Infect. 2022. PMID: 34547457 Free PMC article.
-
Assessment of Neutralizing Antibody Response Against SARS-CoV-2 Variants After 2 to 3 Doses of the BNT162b2 mRNA COVID-19 Vaccine.JAMA Netw Open. 2022 May 2;5(5):e2210780. doi: 10.1001/jamanetworkopen.2022.10780. JAMA Netw Open. 2022. PMID: 35532938 Free PMC article.
-
Immune Responses Induced by mRNA Vaccination in Mice, Monkeys and Humans.Vaccines (Basel). 2021 Jan 18;9(1):61. doi: 10.3390/vaccines9010061. Vaccines (Basel). 2021. PMID: 33477534 Free PMC article. Review.
Cited by
-
T and B cell responses in different immunization scenarios for COVID-19: a narrative review.Front Immunol. 2025 Mar 18;16:1535014. doi: 10.3389/fimmu.2025.1535014. eCollection 2025. Front Immunol. 2025. PMID: 40170841 Free PMC article. Review.
-
Validation of the Enzyme-Linked ImmunoSpot Analytic Method for the Detection of Human IFN-γ from Peripheral Blood Mononuclear Cells in Response to the SARS-CoV-2 Spike Protein.Biomolecules. 2024 Oct 11;14(10):1286. doi: 10.3390/biom14101286. Biomolecules. 2024. PMID: 39456219 Free PMC article.
-
Incidence of dynamic seroconversion in subjects received the first dose of the SARS-COV-2 vaccine (AstraZeneca, Moderna and Pfizer) in Kinshasa, Democratic Republic of Congo: prospective cohort study.BMC Infect Dis. 2025 Mar 11;25(1):342. doi: 10.1186/s12879-025-10754-4. BMC Infect Dis. 2025. PMID: 40069636 Free PMC article.
References
-
- First Covid-19 Vaccine Given to U.S. Public. https://www.wsj.com/articles/covid-19-vaccinations-in-the-u-s-slated-to-.... Accessed 24 October 2022.
-
- FDA Approves First COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/fda-approves-first-c.... Accessed 24 October 2022.
-
- Spikevax and Moderna COVID-19 Vaccine. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed 24 October 2022.
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922. doi: 10.15585/mmwr.mm6950e2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

