Coumarin N-Acylhydrazone Derivatives: Green Synthesis and Antioxidant Potential-Experimental and Theoretical Study
- PMID: 37891938
- PMCID: PMC10604617
- DOI: 10.3390/antiox12101858
Coumarin N-Acylhydrazone Derivatives: Green Synthesis and Antioxidant Potential-Experimental and Theoretical Study
Abstract
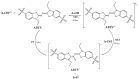
Coumarin N-acylhydrazone derivatives were synthesized in the reaction of 3-acetylcoumarin and different benzohydrazides in the presence of molecular iodine as catalyst and at room temperature. All reactions were rapidly completed, and products were obtained in good to excellent yields. It is important to emphasize that four products were reported for the first time in this study. The obtained compounds were subjected to evaluation of their in vitro antioxidative activity using DPPH, ABTS, and FRAP methods. It was shown that products with a catechol moiety in their structure are the most potent antioxidant agents. The thermodynamic parameters and Gibbs free energies of reactions were used to determine the most probable mechanism of action. The results of in silico examination emphasize the need to take solvent polarity and free radical species into account when examining antiradical action. It was discovered by using computational approaches that HAT and SPLET are competitive molecular pathways for the radical scavenging activity of all compounds in polar mediums, while the HAT is the dominant mechanism in non-polar environments.
Keywords: DFT; antioxidant activity; coumarin N-acylhydrazone derivatives; green synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Murray D.H., Mendez J., Brown S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. J. Wiley & Sons; New York, NY, USA: 1982. p. 1.
-
- O’Kennedy R., Thornes R.D. Coumarins: Biology, Applications and Mode of Action. J. Wiley & Sons; Chichester, UK: 1997. p. 1.
-
- Sharifi-Rad J., Cruz-Martins N., López-Jornet P., Pons-Fuster Lopez E., Harun N., Yeskaliyeva B., Beyatli A., Sytar O., Shaheen S., Sharopov F., et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med. Cell. Longev. 2021;2021:6492346. doi: 10.1155/2021/6492346. - DOI - PMC - PubMed
Grants and funding
- 451-03-47/2023-01/200378/Ministry of Science, Technological Development, and Innovations, Republic of Serbia
- 451-03-47/2023-01/200252/Ministry of Science, Technological Development, and Innovations, Republic of Serbia
- VI-03-232/Project of the Center for Scientific Research of SANU and the University of Kragujevac
LinkOut - more resources
Full Text Sources
Miscellaneous

