Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer's Disease
- PMID: 37892143
- PMCID: PMC10604953
- DOI: 10.3390/biom13101461
Decanoic Acid Rescues Differences in AMPA-Mediated Calcium Rises in Hippocampal CA1 Astrocytes and Neurons in the 5xFAD Mouse Model of Alzheimer's Disease
Abstract
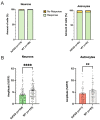
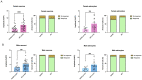
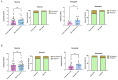
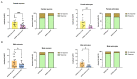
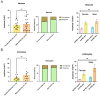
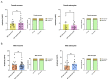
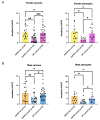
Alzheimer's disease (AD), a devastating neurodegenerative disease characterized by cognitive dysfunctions, is associated with high levels of amyloid beta 42 (Aβ42), which is believed to play a role in cellular damage and signaling changes in AD. Decanoic acid has been shown to be therapeutic in AD. Glutamatergic signaling within neurons and astrocytes of the CA1 region of the hippocampus is critical in cognitive processes, and previous work has indicated deficiencies in this signaling in a mouse model of AD. In this study, we investigated glutamate-mediated signaling by evaluating AMPA-mediated calcium rises in female and male CA1 neurons and astrocytes in a mouse model of AD and examined the potential of decanoic acid to normalize this signaling. In brain slices from 5xFAD mice in which there are five mutations leading to increasing levels of Aβ42, AMPA-mediated calcium transients in CA1 neurons and astrocytes were significantly lower than that seen in wildtype controls in both females and males. Interestingly, incubation of 5xFAD slices in decanoic acid restored AMPA-mediated calcium levels in neurons and astrocytes in both females and males to levels indistinguishable from those seen in wildtype, whereas similar exposure to decanoic acid did not result in changes in AMPA-mediated transients in neurons or astrocytes in either sex in the wildtype. Our data indicate that one mechanism by which decanoic acid could improve cognitive functioning is through normalizing AMPA-mediated signaling in CA1 hippocampal cells.
Keywords: Alzheimer’s disease; calcium; diet; hippocampus; medium-chain fatty acids.
Conflict of interest statement
All authors disclose that they have no relevant financial or non-financial interests to disclose.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

