Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center
- PMID: 37892166
- PMCID: PMC10605275
- DOI: 10.3390/biom13101484
Outcomes and Adverse Effects of Voretigene Neparvovec Treatment for Biallelic RPE65-Mediated Inherited Retinal Dystrophies in a Cohort of Patients from a Single Center
Abstract
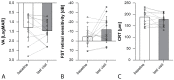
Our study evaluated the morphological and functional outcomes, and the side effects, of voretigene neparvovec (VN) gene therapy for RPE65-mediated inherited retinal dystrophies (IRDs) in 12 eyes (six patients) at the Oxford Eye Hospital with a mean follow-up duration of 8.2 (range 1-12) months. All patients reported a subjective vision improvement 1 month after gene therapy. Best-corrected visual acuity (BCVA) remained stable (baseline: 1.28 (±0.71) vs. last follow-up: 1.46 (±0.60); p = 0.25). Average white Full-Field Stimulus Testing (FST) showed a trend towards improvement (baseline: -4.41 (±10.62) dB vs. last follow-up: -11.98 (±13.83) dB; p = 0.18). No changes in central retinal thickness or macular volume were observed. The side effects included mild intraocular inflammation (two eyes) and cataracts (four eyes). Retinal atrophy occurred in 10 eyes (eight mild, two severe) but did not impact FST measurements during the follow-up period. Increased intraocular pressure (IOP) was noted in three patients (six eyes); four eyes (two patients) required glaucoma surgery. The overall safety and effectiveness of VN treatment in our cohort align with previous VN clinical trials, except for the higher occurrence of retinal atrophy and increased IOP in our cohort. This suggests that raised IOP and retinal atrophy may be more common than previously reported.
Keywords: IRD; RPE65-mediated inherited retinal dystrophies; adverse effects; functional outcomes; gene therapy; high IOP; retinal atrophy; voretigene neparvovec.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sallum J.M.F., Kaur V.P., Shaikh J., Banhazi J., Spera C., Aouadj C., Viriato D., Fischer M.D. Epidemiology of Mutations in the 65-kDa Retinal Pigment Epithelium (RPE65) Gene-Mediated Inherited Retinal Dystrophies: A Systematic Literature Review. Adv. Ther. 2022;39:1179–1198. doi: 10.1007/s12325-021-02036-7. - DOI - PMC - PubMed
-
- Maguire A.M., Russell S., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., Marshall K.A., et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 Mutation-Associated Inherited Retinal Dystrophy: Results of Phase 1 and 3 Trials. Ophthalmology. 2019;126:1273–1285. doi: 10.1016/j.ophtha.2019.06.017. - DOI - PubMed
-
- Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

