5'-tRF-19-Q1Q89PJZ Suppresses the Proliferation and Metastasis of Pancreatic Cancer Cells via Regulating Hexokinase 1-Mediated Glycolysis
- PMID: 37892195
- PMCID: PMC10605356
- DOI: 10.3390/biom13101513
5'-tRF-19-Q1Q89PJZ Suppresses the Proliferation and Metastasis of Pancreatic Cancer Cells via Regulating Hexokinase 1-Mediated Glycolysis
Abstract
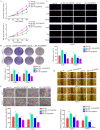
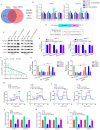
tRNA-derived small RNAs (tDRs) are dysregulated in several diseases, including pancreatic cancer (PC). However, only a limited number of tDRs involved in PC progression are known. Herein, a novel tDR, 5'-tRF-19-Q1Q89PJZ (tRF-19-Q1Q89PJZ), was verified in PC plasma using RNA and Sanger sequencing. tRF-19-Q1Q89PJZ was downregulated in PC tissues and plasma, which was related to advanced clinical characteristics and poor prognosis. tRF-19-Q1Q89PJZ overexpression inhibited the malignant activity of PC cells in vitro, while tRF-19-Q1Q89PJZ inhibition produced an opposite effect. The differentially expressed genes induced by tRF-19-Q1Q89PJZ overexpression were enriched in "pathways in cancer" and "glycolysis". Mechanistically, tRF-19-Q1Q89PJZ directly sponged hexokinase 1 (HK1) mRNA and inhibited its expression, thereby suppressing glycolysis in PC cells. HK1 restoration relieved the inhibitory effect of tRF-19-Q1Q89PJZ on glycolysis in PC cells and on their proliferation and mobility in vitro. tRF-19-Q1Q89PJZ upregulation inhibited PC cell proliferation and metastasis in vivo and suppressed HK1 expression in tumor tissues. Furthermore, tRF-19-Q1Q89PJZ expression was attenuated under hypoxia. Collectively, these findings indicate that tRF-19-Q1Q89PJZ suppresses the malignant activity of PC cells by regulating HK1-mediated glycolysis. Thus, tRF-19-Q1Q89PJZ may serve as a key target for PC therapy.
Keywords: 5′-tRF-19-Q1Q89PJZ; glycolysis; hexokinase 1; metastasis; pancreatic cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hue J.J., Bingmer K., Sugumar K., Ocuin L.M., Rothermel L.D., Winter J.M., Ammori J.B., Hardacre J.M. Mortality and Survival Among Octogenarians with Localized Pancreatic Head Cancer: A National Cancer Database Analysis. J. Gastrointest. Surg. 2021;25:2582–2592. doi: 10.1007/s11605-021-04949-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

