Navigating the ERK1/2 MAPK Cascade
- PMID: 37892237
- PMCID: PMC10605237
- DOI: 10.3390/biom13101555
Navigating the ERK1/2 MAPK Cascade
Abstract
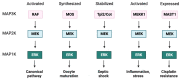
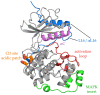
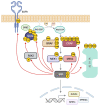
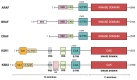
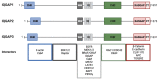
The RAS-ERK pathway is a fundamental signaling cascade crucial for many biological processes including proliferation, cell cycle control, growth, and survival; common across all cell types. Notably, ERK1/2 are implicated in specific processes in a context-dependent manner as in stem cells and pancreatic β-cells. Alterations in the different components of this cascade result in dysregulation of the effector kinases ERK1/2 which communicate with hundreds of substrates. Aberrant activation of the pathway contributes to a range of disorders, including cancer. This review provides an overview of the structure, activation, regulation, and mutational frequency of the different tiers of the cascade; with a particular focus on ERK1/2. We highlight the importance of scaffold proteins that contribute to kinase localization and coordinate interaction dynamics of the kinases with substrates, activators, and inhibitors. Additionally, we explore innovative therapeutic approaches emphasizing promising avenues in this field.
Keywords: ERK1/2; MAPKs; cancer; inhibitors; scaffold; therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

