Administration of Linoleoylethanolamide Reduced Weight Gain, Dyslipidemia, and Inflammation Associated with High-Fat-Diet-Induced Obesity
- PMID: 37892524
- PMCID: PMC10609991
- DOI: 10.3390/nu15204448
Administration of Linoleoylethanolamide Reduced Weight Gain, Dyslipidemia, and Inflammation Associated with High-Fat-Diet-Induced Obesity
Abstract
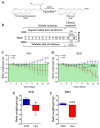
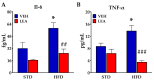
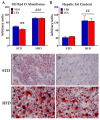
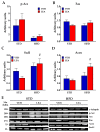
Acylethanolamides (NAEs) are bioactive lipids derived from diet fatty acids that modulate important homeostatic functions, including appetite, fatty acid synthesis, mitochondrial respiration, inflammation, and nociception. Among the naturally circulating NAEs, the pharmacology of those derived from either arachidonic acid (Anandamide), oleic acid (OEA), and palmitic acid (PEA) have been extensively characterized in diet-induced obesity. For the present work, we extended those studies to linoleoylethanolamide (LEA), one of the most abundant NAEs found not only in plasma and body tissues but also in foods such as cereals. In our initial study, circulating concentrations of LEA were found to be elevated in overweight humans (body mass index (BMI, Kg/m2) > 25) recruited from a representative population from the south of Spain, together with AEA and the endocannabinoid 2-Arachidonoyl glycerol (2-AG). In this population, LEA concentrations correlated with the circulating levels of cholesterol and triglycerides. In order to gain insight into the pharmacology of LEA, we administered it for 14 days (10 mg/kg i.p. daily) to obese male Sprague Dawley rats receiving a cafeteria diet or a standard chow diet for 12 consecutive weeks. LEA treatment resulted in weight loss and a reduction in circulating triglycerides, cholesterol, and inflammatory markers such as Il-6 and Tnf-alpha. In addition, LEA reduced plasma transaminases and enhanced acetyl-CoA-oxidase (Acox) and Uncoupling protein-2 (Ucp2) expression in the liver of the HFD-fed animals. Although the liver steatosis induced by the HFD was not reversed by LEA, the overall data suggest that LEA contributes to the homeostatic signals set in place in response to diet-induced obesity, potentially contributing with OEA to improve lipid metabolism after high fat intake. The anti-inflammatory response associated with its administration suggests its potential for use as a nutrient supplement in non-alcoholic steatohepatitis.
Keywords: acylethanolamides; high-fat diet; linoleic acid; linoleylethanolamide; liver steatosis; obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

