Mapping the Most Common Founder Variant in RSPH9 That Causes Primary Ciliary Dyskinesia in Multiple Consanguineous Families of Bedouin Arabs
- PMID: 37892643
- PMCID: PMC10607267
- DOI: 10.3390/jcm12206505
Mapping the Most Common Founder Variant in RSPH9 That Causes Primary Ciliary Dyskinesia in Multiple Consanguineous Families of Bedouin Arabs
Abstract
Introduction: Primary ciliary dyskinesia (PCD) is a congenital thoracic disorder caused by dysfunction of motile cilia, resulting in insufficient mucociliary clearance of the lungs. The overall aim of this study is to identify causative defective genes in PCD-affected individuals in the Kuwaiti population.
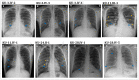
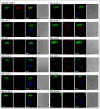
Methods: A cohort of multiple consanguineous PCD families was identified from Kuwaiti patients and genomic DNA from the family members was isolated using standard procedures. The DNA samples from all affected individuals were analyzed by whole exome sequencing (WES). Transmission electron microscopy (TEM) and immunofluorescent analysis (IF) were performed on samples obtained by nasal brushings to identify specific structural abnormalities within ciliated cells.
Results: Here, we present six multiplex families with 11 patients who all presented with typical PCD symptoms. Ten out of eleven patients inherited a 3 bp homozygous deletion of GAA in RSPH9, whereas the eleventh patients inherited this variant in trans with a frameshift deletion in RSPH9. Genetic results were confirmed by segregation analysis. The in-frame deletion of GAA in RSPH9 has previously been published as pathogenic in both annotated RSPH9 transcript variants (1 and 2). In contrast, the previously unpublished RSPH9 frameshift deletion identified in KU-15.IV2 impacts only RSPH9 transcript variant two. Regarding all 11 PCD individuals analyzed, IF results demonstrated absence of RSPH9 protein and TEM analysis showed the typical findings in RSPH9 mutant individuals.
Conclusions: We present the largest cohort of PCD individuals affected by the founder in-frame deletion GAA in RSPH9. This founder variant is the most common PCD-causing variant in Bedouin Arabs in Kuwait.
Keywords: RSPH9; consanguinity; genetics of ciliopathy; primary ciliary dyskinesia; pulmonary diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hannah W.B., Seifert B.A., Truty R., Zariwala M.A., Ameel K., Zhao Y., Nykamp K., Gaston B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: A genetic database analysis. Lancet Respir. Med. 2022;10:459–468. doi: 10.1016/S2213-2600(21)00453-7. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

