RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia
- PMID: 37892756
- PMCID: PMC10607860
- DOI: 10.3390/jcm12206618
RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia
Abstract
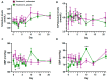
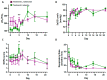
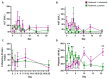
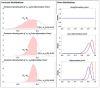
Even though SARS-CoV-2 was declared by WHO as constituting no longer a public health emergency, the development of effective treatments against SARS-CoV-2 infection remains a critical issue to prevent complications, particularly in fragile patients. The protease inhibitor nafamostat, currently used in Japan and Korea for pancreatitis, owing to its anticoagulant properties for disseminated intravascular coagulation (DIC), is appealing for the treatment of COVID-19 infection, because it potently inhibits the transmembrane protease serine 2 (TMPRSS2) that, after virus binding to ACE-2, allows virus entry into the cells and replication. Moreover, it could prevent the DIC and pulmonary embolism frequently associated with COVID-19 infection. The goal of the RAndomized Clinical Trial Of NAfamostat (RACONA) study, designed as a prospective randomized, double-blind placebo-controlled clinical trial, was to investigate the efficacy and safety of nafamostat mesylate (0.10 mg/kg/h iv for 7 days), on top of the optimal treatment, in COVID-19 hospitalized patients. We could screen 131 patients, but due to the predefined strict inclusion and exclusion criteria, only 15 could be randomized to group 1 (n = 7) or group 2 (n = 8). The results of an ad interim safety analysis showed similar overall trends for variables evaluating renal function, coagulation, and inflammation. No adverse events, including hyperkalemia, were found to be associated with nafamostat. Thus, the RACONA study showed a good safety profile of nafamostat, suggesting that it could be usefully used in COVID-19 hospitalized patients.
Keywords: COVID-19; SARS-CoV-2; coagulation; nafamostat mesylate; safety; transmembrane protease serine 2 TMPRSS2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Isgrò M.A., Trillò G., Russo L., Tornesello A.L., Buonaguro L., Tornesello M.L., Miscio L., Normanno N., Bianchi A.A.M., Buonaguro F.M., et al. Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT—IRCCS “Fondazione Pascale” Cancer Center (Naples, Italy) Infect. Agents Cancer. 2022;17:40. doi: 10.1186/s13027-022-00451-1. - DOI - PMC - PubMed
-
- Chuenkitmongkol S., Solante R., Burhan E., Chariyalertsak S., Chiu N.-C., Do-Van D., Masliyana H., Kao-Pin H., Sasisopin K., Prasad S.K. Expert review on global real-world vaccine effectiveness against SARS-CoV-2. Expert. Rev. Vaccines. 2022;21:1255–1268. doi: 10.1080/14760584.2022.2092472. - DOI - PubMed
-
- Botton J., Jabagi M.J., Bertrand M., Baricault B., Drouin J., le Vu S., Weill A., Farrington P., Zureik M., Dray-Spira R. Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France. Ann. Intern. Med. 2022;175:1250–1257. doi: 10.7326/M22-0988. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

