Whole Liver Derived Acellular Extracellular Matrix for Bioengineering of Liver Constructs: An Updated Review
- PMID: 37892856
- PMCID: PMC10604736
- DOI: 10.3390/bioengineering10101126
Whole Liver Derived Acellular Extracellular Matrix for Bioengineering of Liver Constructs: An Updated Review
Abstract
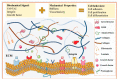
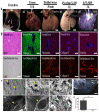
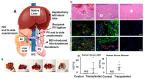
Biomaterial templates play a critical role in establishing and bioinstructing three-dimensional cellular growth, proliferation and spatial morphogenetic processes that culminate in the development of physiologically relevant in vitro liver models. Various natural and synthetic polymeric biomaterials are currently available to construct biomimetic cell culture environments to investigate hepatic cell-matrix interactions, drug response assessment, toxicity, and disease mechanisms. One specific class of natural biomaterials consists of the decellularized liver extracellular matrix (dECM) derived from xenogeneic or allogeneic sources, which is rich in bioconstituents essential for the ultrastructural stability, function, repair, and regeneration of tissues/organs. Considering the significance of the key design blueprints of organ-specific acellular substrates for physiologically active graft reconstruction, herein we showcased the latest updates in the field of liver decellularization-recellularization technologies. Overall, this review highlights the potential of acellular matrix as a promising biomaterial in light of recent advances in the preparation of liver-specific whole organ scaffolds. The review concludes with a discussion of the challenges and future prospects of liver-specific decellularized materials in the direction of translational research.
Keywords: decellularization; liver; recellularization; scaffolds; tissue and organoids.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

