YAP/ACSL4 Pathway-Mediated Ferroptosis Promotes Renal Fibrosis in the Presence of Kidney Stones
- PMID: 37893066
- PMCID: PMC10603838
- DOI: 10.3390/biomedicines11102692
YAP/ACSL4 Pathway-Mediated Ferroptosis Promotes Renal Fibrosis in the Presence of Kidney Stones
Abstract
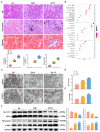
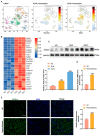
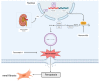
The potential association between calcium oxalate stones and renal fibrosis has been extensively investigated; however, the underlying mechanisms remain unclear. Ferroptosis is a novel form of cell death characterized by iron-dependent lipid peroxidation and regulated by acyl coenzyme A synthase long-chain family member 4 (ACSL4). Yes-associated protein (YAP), a transcriptional co-activator in the Hippo pathway, promotes ferroptosis by modulating ACSL4 expression. Nevertheless, the involvement of YAP-ACSL4 axis-mediated ferroptosis in calcium oxalate crystal deposition-induced renal fibrosis and its molecular mechanisms have not been elucidated. In this study, we investigated ACSL4 expression and ferroptosis activation in the kidney tissues of patients with calcium oxalate stones and in mice using single-cell sequencing, transcriptome RNA sequencing, immunohistochemical analysis, and Western blot analysis. In vivo and in vitro experiments demonstrated that inhibiting ferroptosis or ACSL4 mitigated calcium oxalate crystal-induced renal fibrosis. Furthermore, YAP expression was elevated in the kidney tissues of patients with calcium oxalate stones and in calcium oxalate crystal-stimulated human renal tubular epithelial cell lines. Mechanistically, in calcium oxalate crystal-stimulated human renal tubular epithelial cell lines, activated YAP translocated to the nucleus and enhanced ACSL4 expression, consequently inducing cellular ferroptosis. Moreover, YAP silencing suppressed ferroptosis by downregulating ACSL4 expression, thereby attenuating calcium oxalate crystal-induced renal fibrosis. Conclusively, our findings suggest that YAP-ACSL4-mediated ferroptosis represents an important mechanism underlying the induction of renal fibrosis by calcium oxalate crystal deposition. Targeting the YAP-ACSL4 axis and ferroptosis may therefore hold promise as a potential therapeutic approach for preventing renal fibrosis in patients with kidney stones.
Keywords: YAP–ACSL4 axis; calcium oxalate crystal; ferroptosis; kidney stone; renal fibrosis.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures




References
-
- Lu H., Sun X., Jia M., Sun F., Zhu J., Chen X., Chen K., Jiang K. Rosiglitazone Suppresses Renal Crystal Deposition by Ameliorating Tubular Injury Resulted from Oxidative Stress and Inflammatory Response via Promoting the Nrf2/HO-1 Pathway and Shifting Macrophage Polarization. Oxid. Med. Cell Longev. 2021;2021:5527137. doi: 10.1155/2021/5527137. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

