The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development
- PMID: 37893069
- PMCID: PMC10603948
- DOI: 10.3390/biomedicines11102695
The RELT Family of Proteins: An Increasing Awareness of Their Importance for Cancer, the Immune System, and Development
Abstract
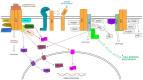
This review highlights Receptor Expressed in Lymphoid Tissues (RELT), a Tumor Necrosis Factor Superfamily member, and its two paralogs, RELL1 and RELL2. Collectively, these three proteins are referred to as RELTfms and have gained much interest in recent years due to their association with cancer and other human diseases. A thorough knowledge of their physiological functions, including the ligand for RELT, is lacking, yet emerging evidence implicates RELTfms in a variety of processes including cytokine signaling and pathways that either promote cell death or survival. T cells from mice lacking RELT exhibit increased responses against tumors and increased inflammatory cytokine production, and multiple lines of evidence indicate that RELT may promote an immunosuppressive environment for tumors. The relationship of individual RELTfms in different cancers is not universal however, as evidence indicates that individual RELTfms may be risk factors in certain cancers yet appear to be protective in other cancers. RELTfms are important for a variety of additional processes related to human health including microbial pathogenesis, inflammation, behavior, reproduction, and development. All three proteins have been strongly conserved in all vertebrates, and this review aims to provide a clearer understanding of the current knowledge regarding these interesting proteins.
Keywords: RELL1; RELL2; RELT; TNFRSF; apoptosis; cancer; cytokine signaling; immune cells; inflammation; interferon.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

