TCR-Engineered Lymphocytes Targeting NY-ESO-1: In Vitro Assessment of Cytotoxicity against Tumors
- PMID: 37893178
- PMCID: PMC10604587
- DOI: 10.3390/biomedicines11102805
TCR-Engineered Lymphocytes Targeting NY-ESO-1: In Vitro Assessment of Cytotoxicity against Tumors
Abstract
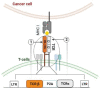
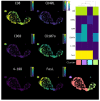
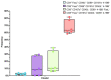
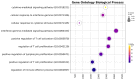
Adoptive T-cell therapies tailored for the treatment of solid tumors encounter intricate challenges, necessitating the meticulous selection of specific target antigens and the engineering of highly specific T-cell receptors (TCRs). This study delves into the cytotoxicity and functional characteristics of in vitro-cultured T-lymphocytes, equipped with a TCR designed to precisely target the cancer-testis antigen NY-ESO-1. Flow cytometry analysis unveiled a notable increase in the population of cells expressing activation markers upon encountering the NY-ESO-1-positive tumor cell line, SK-Mel-37. Employing the NanoString platform, immune transcriptome profiling revealed the upregulation of genes enriched in Gene Ontology Biological Processes associated with the IFN-γ signaling pathway, regulation of T-cell activation, and proliferation. Furthermore, the modified T cells exhibited robust cytotoxicity in an antigen-dependent manner, as confirmed by the LDH assay results. Multiplex immunoassays, including LEGENDplex™, additionally demonstrated the elevated production of cytotoxicity-associated cytokines driven by granzymes and soluble Fas ligand (sFasL). Our findings underscore the specific targeting potential of engineered TCR T cells against NY-ESO-1-positive tumors. Further comprehensive in vivo investigations are essential to thoroughly validate these results and effectively harness the intrinsic potential of genetically engineered T cells for combating cancer.
Keywords: NY-ESO-1; T-cell activation; TCR; TCR-T cells; cellular cytotoxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


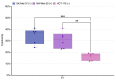
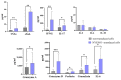
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

