Commercially Accessible High-Performance Aluminum-Air Battery Cathodes through Electrodeposition of Mn and Ni Species on Fuel Cell Cathodes
- PMID: 37893367
- PMCID: PMC10609553
- DOI: 10.3390/mi14101930
Commercially Accessible High-Performance Aluminum-Air Battery Cathodes through Electrodeposition of Mn and Ni Species on Fuel Cell Cathodes
Abstract
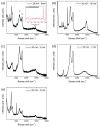
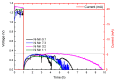
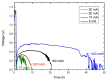
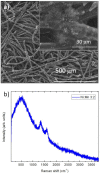
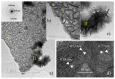
This study presents a cost-effective method for producing high-performance cathodes for aluminum-air batteries. Commercial fuel cell cathodes are modified through electrodeposition of nickel and manganese species. The optimal conditions for electrodeposition are determined using a combination of structural (Raman, SEM, TEM) and electrochemical (LSV, EI, discharge curves) characterization techniques. The structural analysis confirms successful incorporation of nickel and manganese species onto the cathode surface. Electrochemical tests demonstrate enhanced electrochemical activity compared to unmodified cathodes. By combining the favorable properties of electrodeposited manganese species with nickel species, a high-performance cathode is obtained. The developed cathode exhibits capacities of 50 mA h cm-2 in aluminum-air batteries across a wide range of current densities. The electrodeposition method proves effective in improving electrochemical performance. A key advantage of this method is its simplicity and cost-effectiveness. The use of commercially available materials and well-established electrodeposition techniques allows for easy scalability and commercialization. This makes it a viable option for large-scale production of high-performance cathodes for the next-generation energy storage devices.
Keywords: Al-air; electrodeposition; metal-air batteries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Liu Y., Sun Q., Li W., Adair K.R., Li J., Sun X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017;2:246–277. doi: 10.1016/j.gee.2017.06.006. - DOI
-
- Elia G.A., Kravchyk K.V., Kovalenko M.V., Chacón J., Holland A., Wills R.G. An overview and prospective on Al and Al-ion battery technologies. J. Power Sources. 2021;481:228870. doi: 10.1016/j.jpowsour.2020.228870. - DOI
-
- Martin J.J., Neburchilov V., Wang H., Qu W. Air cathodes for metal-air batteries and fuel cells; Proceedings of the 2009 IEEE Electrical Power & Energy Conference (EPEC); Montreal, QC, Canada. 22–23 October 2009; pp. 1–6. - DOI
-
- Matsuki K., Kamada H. Oxygen reduction electrocatalysis on some manganese oxides. Electrochim. Acta. 1986;31:13–18. doi: 10.1016/0013-4686(86)80054-8. - DOI
-
- Wei S., Liu H., Wei R., Chen L. Cathodes with MnO2 catalysts for metal fuel battery. Front. Energy. 2019;13:9–15. doi: 10.1007/s11708-019-0611-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

