Thermal Inactivation of Hepatitis E Virus in Pork Products Estimated with a Semiquantitative Infectivity Assay
- PMID: 37894109
- PMCID: PMC10609450
- DOI: 10.3390/microorganisms11102451
Thermal Inactivation of Hepatitis E Virus in Pork Products Estimated with a Semiquantitative Infectivity Assay
Abstract
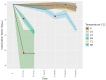
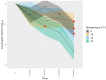
Hepatitis E virus genotype 3 (HEV-3) is a food-borne pathogen causative of hepatitis E infections in humans. In Europe, HEV-3 is mainly transmitted through the consumption of raw or undercooked pork. In order to determine the effectiveness of control measures that can be taken in the industry or by the consumer, it is pivotal to determine the infectivity of HEV present in pork products after thermal food-processing steps. First, we implemented a method for the detection of infectious HEV-3c and HEV-3e in a cell culture medium and in extracts from inoculated pork products. Next, we investigated the effect of the thermal inactivation of HEV by mimicking food-processing steps specific for dried sausage and liver homogenate matrices. After four weeks, HEV-inoculated dried sausage subjected to 21 °C or lower temperatures was still infectious. For the liver homogenate, the highest HEV-3c/e inactivation of the conditions tested was observed at 71 °C for five min or longer. Finally, our method was able to successfully detect and estimate viral loads of infectious HEV in naturally infected pig livers. Our data provide a basis for the future use of the quantitative microbial risk assessment of infectious HEV in pork products that are subjected to thermal food processing steps.
Keywords: HEV infectivity; HEV-3c; HEV-3e; cell culture method; food processing; food-borne; immunofluorescence; pork matrices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hazards E.P.O.B., Ricci A., Allende A., Bolton D., Chemaly M., Davies R., Fernandez Escamez P.S., Herman L., Koutsoumanis K., Lindqvist R., et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017;15:e04886. doi: 10.2903/j.efsa.2017.4886. - DOI - PMC - PubMed
-
- Adlhoch C., Avellon A., Baylis S.A., Ciccaglione A.R., Couturier E., de Sousa R., Epstein J., Ethelberg S., Faber M., Feher A., et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J. Clin. Virol. 2016;82:9–16. doi: 10.1016/j.jcv.2016.06.010. - DOI - PubMed
-
- Aspinall E.J., Couturier E., Faber M., Said B., Ijaz S., Tavoschi L., Takkinen J., Adlhoch C., The Country E. Hepatitis E virus infection in Europe: Surveillance and descriptive epidemiology of confirmed cases, 2005 to 2015. Euro. Surveill. 2017;22:30561. doi: 10.2807/1560-7917.ES.2017.22.26.30561. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

