The Multifaceted Functions of Prion Protein (PrPC) in Cancer
- PMID: 37894349
- PMCID: PMC10605613
- DOI: 10.3390/cancers15204982
The Multifaceted Functions of Prion Protein (PrPC) in Cancer
Abstract
The cellular prion protein (PrPC) is a glycoprotein anchored to the cell surface by glycosylphosphatidylinositol (GPI). PrPC is expressed both in the brain and in peripheral tissues. Investigations on PrPC's functions revealed its direct involvement in neurodegenerative and prion diseases, as well as in various physiological processes such as anti-oxidative functions, copper homeostasis, trans-membrane signaling, and cell adhesion. Recent findings have revealed the ectopic expression of PrPC in various cancers including gastric, melanoma, breast, colorectal, pancreatic, as well as rare cancers, where PrPC promotes cellular migration and invasion, tumor growth, and metastasis. Through its downstream signaling, PrPC has also been reported to be involved in resistance to chemotherapy and tumor cell apoptosis. This review summarizes the variance of expression of PrPC in different types of cancers and discusses its roles in their development and progression, as well as its use as a potential target to treat such cancers.
Keywords: cancer; drug resistance; prion protein (PrPC); therapeutic target.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
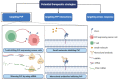
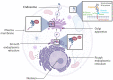
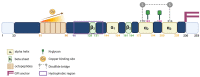
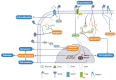
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

