Endothelialization of Whey Protein Isolate-Based Scaffolds for Tissue Regeneration
- PMID: 37894531
- PMCID: PMC10609092
- DOI: 10.3390/molecules28207052
Endothelialization of Whey Protein Isolate-Based Scaffolds for Tissue Regeneration
Abstract
Background: Whey protein isolate (WPI) is a by-product from the dairy industry, whose main component is β-lactoglobulin. Upon heating, WPI forms a hydrogel which can both support controlled drug delivery and enhance the proliferation and osteogenic differentiation of bone-forming cells. This study makes a novel contribution by evaluating the ability of WPI hydrogels to support the growth of endothelial cells, which are essential for vascularization, which in turn is a pre-requisite for bone regeneration.
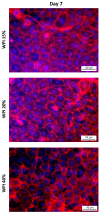
Methods: In this study, the proliferation and antioxidant levels in human umbilical vascular endothelial cells (HUVECs) cultured with WPI supplementation were evaluated using real-time cell analysis and flow cytometry. Further, the attachment and growth of HUVECs seeded on WPI-based hydrogels with different concentrations of WPI (15%, 20%, 30%, 40%) were investigated.
Results: Supplementation with WPI did not affect the viability or proliferation of HUVECs monitored with real-time cell analysis. At the highest used concentration of WPI (500 µg/mL), a slight induction of ROS production in HUVECs was detected as compared with control samples, but it was not accompanied by alterations in cellular thiol levels. Regarding WPI-based hydrogels, HUVEC adhered and spread on all samples, showing good metabolic activity. Notably, cell number was highest on samples containing 20% and 30% WPI.
Conclusions: The demonstration of the good compatibility of WPI hydrogels with endothelial cells in these experiments is an important step towards promoting the vascularization of hydrogels upon implantation in vivo, which is expected to improve implant outcomes in the future.
Keywords: 3D cell seeding; endothelial cell compatibility; hydrogels; thiol levels; tubular scaffolds; whey protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

