Montmorillonite Catalyzed Synthesis of Novel Steroid Dimers
- PMID: 37894547
- PMCID: PMC10609449
- DOI: 10.3390/molecules28207068
Montmorillonite Catalyzed Synthesis of Novel Steroid Dimers
Abstract
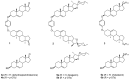
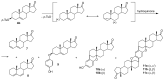
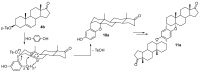
The reactions of sterols (androst-5-en-3β-ol-17-one, diosgenin, and cholesterol) and their tosylates with hydroquinone aimed at the synthesis of O,O-1,4-phenylene-linked steroid dimers were studied. The reaction course strongly depended on the conditions used. The study has shown that the major reaction products are the elimination products and unusual steroid dimers resulting from the nucleophilic attack of the hydroquinone C2 carbon atom on the steroid C3 position, followed by an intramolecular addition to the C5-C6 double bond. A different reaction course was observed when montmorillonite K10 was used as a catalyst. The reaction of androst-5-en-3β-ol-17-one under the promotion of this catalyst afforded the O,O-1,4-phenylene-linked steroid dimer in addition to the disteroidal ether. The formation of the latter compound was suppressed by using 3-tosylate as a substrate instead of the free sterol. The reactions of androst-5-en-3β-ol-17-one tosylate and cholesteryl tosylate with hydroquinone catalyzed by montmorillonite K10 carried out under optimized conditions afforded the desired dimers in 31% and 67% yield, respectively.
Keywords: 1,4-phenylene-linked dimers; DHEA; cholesterol; montmorillonite; steroid dimers; steroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


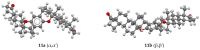
References
-
- Nahar L., Sarker S.D. Steroid Dimers—Chemistry and Applications in Drug Design and Delivery. John Wiley & Sons; Chichester, UK: 2012.
-
- Banerji J., Chatterjee A., Itoh Y., Kikuchi T. New steroid alkaloids from Chonemorpha macrophylla G. don (Chonemorpha fragrans Moon Alston) Indian J. Chem. 1973;11:1056–1057. ISSN/ISBN 0019-5103.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

