Enantiomeric Complexes Based on Ruthenium(III) and 2,2'-Biimidazole: X-ray Structure and Magnetic Properties
- PMID: 37894692
- PMCID: PMC10609436
- DOI: 10.3390/molecules28207213
Enantiomeric Complexes Based on Ruthenium(III) and 2,2'-Biimidazole: X-ray Structure and Magnetic Properties
Abstract
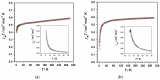
We have prepared and characterized two Ru(III) compounds based on the 2,2'-biimidazole (H2biim) ligand, namely, a single complex of formula cis-[RuCl2(H2biim)2]Cl·4H2O (1) and a racemic mixture of formula {cis-[RuCl2(H2biim)2]Cl}2·4H2O (2), which contains 50% of Ru(III) complex 1. Both compounds crystallize in the monoclinic system with space groups C2 and P21 for 1 and 2, respectively. These complexes exhibit the metal ion bonded to four nitrogen atoms from two H2biim molecules and two chloride ions, which balance part of the positive charges in a distorted octahedral geometry. Significant differences are observed in their crystal packing, which leads to the observation of differences in their respective magnetic behaviors. Despite having imidazole rings in both compounds, π-π stacking interactions occur only in the crystal structure of 2, and the shortest intermolecular Ru···Ru separation in 2 is consequently shorter than that in 1. Variable-temperature dc magnetic susceptibility measurements performed on polycrystalline samples of 1 and 2 reveal different magnetic behaviors at low temperatures: while 1 behaves pretty much as a magnetically isolated mononuclear Ru(III) complex with S = 1/2, 2 exhibits the behavior of an antiferromagnetically coupled system with S = 0 and a maximum in the magnetic susceptibility curve at approximately 3.0 K.
Keywords: 2,2′-biimidazole; crystal explorer; crystal structures; enantiomers; magnetic properties; ruthenium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Bruneau C., Achard M. Allylic ruthenium(IV) complexes in catalysis. Coord. Chem. Rev. 2012;256:525–536. doi: 10.1016/j.ccr.2011.10.018. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

