Rhodamine-Based Cyclic Hydroxamate as Fluorescent pH Probe for Imaging of Lysosomes
- PMID: 37894759
- PMCID: PMC10606023
- DOI: 10.3390/ijms242015073
Rhodamine-Based Cyclic Hydroxamate as Fluorescent pH Probe for Imaging of Lysosomes
Abstract
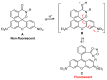
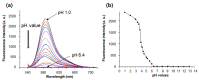
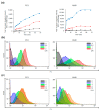
Monitoring the microenvironment within specific cellular regions is crucial for a comprehensive understanding of life events. Fluorescent probes working in different ranges of pH regions have been developed for the local imaging of different pH environments. Especially, rhodamine-based fluorescent pH probes have been of great interest due to their ON/OFF fluorescence depending on the spirolactam ring's opening/closure. By introducing the N-alkyl-hydroxamic acid instead of the alkyl amines in the spirolactam of rhodamine, we were able to tune the pH range where the ring opening and closing of the spirolactam occurs. This six-membered cyclic hydroxamate spirolactam ring of rhodamine B proved to be highly fluorescent in acidic pH environments. In addition, we could monitor pH changes of lysosomes in live cells and zebrafish.
Keywords: acidic pH; fluorescent imaging; fluorescent probe; hydroxamate; rhodamine B.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






Similar articles
-
Comparison of rhodamine 6G, rhodamine B and rhodamine 101 spirolactam based fluorescent probes: A case of pH detection.Spectrochim Acta A Mol Biomol Spectrosc. 2022 Mar 5;268:120662. doi: 10.1016/j.saa.2021.120662. Epub 2021 Nov 26. Spectrochim Acta A Mol Biomol Spectrosc. 2022. PMID: 34865976
-
Fluorescent probes with high pKa values based on traditional, near-infrared rhodamine, and hemicyanine fluorophores for sensitive detection of lysosomal pH variations.Methods. 2019 Sep 15;168:40-50. doi: 10.1016/j.ymeth.2019.07.012. Epub 2019 Jul 22. Methods. 2019. PMID: 31344405 Free PMC article.
-
Design of an Acidic pH-Activated NIR Fluorescent Convertible Rhodamine-Hemicyanine Probe-Peptide Conjugate for Living Cancer Cell Active Targeted Selective Tracking of Lysosomes.Chemistry. 2024 Sep 11;30(51):e202402146. doi: 10.1002/chem.202402146. Epub 2024 Aug 21. Chemistry. 2024. PMID: 38923172
-
A lysosome-targeting rhodamine fluorescent probe for Cu2+ detection and its applications in test kits and zebrafish imaging.Spectrochim Acta A Mol Biomol Spectrosc. 2025 Jan 15;325:125154. doi: 10.1016/j.saa.2024.125154. Epub 2024 Sep 16. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 39316859
-
A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions.Chem Soc Rev. 2008 Aug;37(8):1465-72. doi: 10.1039/b802497a. Epub 2008 Jun 23. Chem Soc Rev. 2008. PMID: 18648672 Review.
Cited by
-
Fluorescent probes in autoimmune disease research: current status and future prospects.J Transl Med. 2025 Apr 9;23(1):411. doi: 10.1186/s12967-025-06430-5. J Transl Med. 2025. PMID: 40205498 Free PMC article. Review.
-
Application of Intelligent Response Fluorescent Probe in Breast Cancer.Molecules. 2024 Sep 10;29(18):4294. doi: 10.3390/molecules29184294. Molecules. 2024. PMID: 39339288 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

