Endotenon-Derived Type II Tendon Stem Cells Have Enhanced Proliferative and Tenogenic Potential
- PMID: 37894787
- PMCID: PMC10606148
- DOI: 10.3390/ijms242015107
Endotenon-Derived Type II Tendon Stem Cells Have Enhanced Proliferative and Tenogenic Potential
Abstract
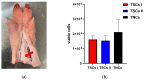
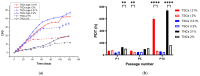
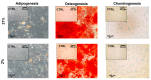
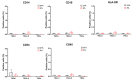
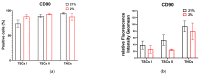
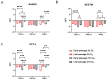
Tendon injuries caused by overuse or age-related deterioration are frequent. Incomplete knowledge of somatic tendon cell biology and their progenitors has hindered interventions for the effective repair of injured tendons. Here, we sought to compare and contrast distinct tendon-derived cell populations: type I and II tendon stem cells (TSCs) and tenocytes (TNCs). Porcine type I and II TSCs were isolated via the enzymatic digestion of distinct membranes (paratenon and endotenon, respectively), while tenocytes were isolated through an explant method. Resultant cell populations were characterized by morphology, differentiation, molecular, flow cytometry, and immunofluorescence analysis. Cells were isolated, cultured, and evaluated in two alternate oxygen concentrations (physiological (2%) and air (21%)) to determine the role of oxygen in cell biology determination within this relatively avascular tissue. The different cell populations demonstrated distinct proliferative potential, morphology, and transcript levels (both for tenogenic and stem cell markers). In contrast, all tendon-derived cell populations displayed multipotent differentiation potential and immunophenotypes (positive for CD90 and CD44). Type II TSCs emerged as the most promising tendon-derived cell population for expansion, given their enhanced proliferative potential, multipotency, and maintenance of a tenogenic profile at early and late passage. Moreover, in all cases, physoxia promoted the enhanced proliferation and maintenance of a tenogenic profile. These observations help shed light on the biological mechanisms of tendon cells, with the potential to aid in the development of novel therapeutic approaches for tendon disorders.
Keywords: TNCs; TSCs; tendinopathies; tendon regeneration; tenogenic markers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Tenogenic Cues Are Biochemically and Environmentally Distinct for Tendon Stem Cells and Mesenchymal/Stromal Stem Cells.Stem Cells Int. 2025 May 13;2025:9047956. doi: 10.1155/sci/9047956. eCollection 2025. Stem Cells Int. 2025. PMID: 40395976 Free PMC article.
-
Characterization of differential properties of rabbit tendon stem cells and tenocytes.BMC Musculoskelet Disord. 2010 Jan 18;11:10. doi: 10.1186/1471-2474-11-10. BMC Musculoskelet Disord. 2010. PMID: 20082706 Free PMC article.
-
A comparison of the stem cell characteristics of murine tenocytes and tendon-derived stem cells.BMC Musculoskelet Disord. 2018 Apr 12;19(1):116. doi: 10.1186/s12891-018-2038-2. BMC Musculoskelet Disord. 2018. PMID: 29650048 Free PMC article.
-
Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells.Acta Biomater. 2022 Jun;145:25-42. doi: 10.1016/j.actbio.2022.04.028. Epub 2022 Apr 22. Acta Biomater. 2022. PMID: 35470075 Review.
-
Tenogenic differentiation of stem cells for tendon repair-what is the current evidence?J Tissue Eng Regen Med. 2011 Aug;5(8):e144-63. doi: 10.1002/term.424. Epub 2011 May 5. J Tissue Eng Regen Med. 2011. PMID: 21548133 Review.
Cited by
-
Tenogenic Cues Are Biochemically and Environmentally Distinct for Tendon Stem Cells and Mesenchymal/Stromal Stem Cells.Stem Cells Int. 2025 May 13;2025:9047956. doi: 10.1155/sci/9047956. eCollection 2025. Stem Cells Int. 2025. PMID: 40395976 Free PMC article.
References
-
- Ciardulli M.C., Lovecchio J., Scala P., Lamparelli E.P., Dale T.P., Giudice V., Giordano E., Selleri C., Forsyth N.R., Maffulli N., et al. 3D Biomimetic Scaffold for Growth Factor Controlled Delivery: An In-Vitro Study of Tenogenic Events on Wharton’s Jelly Mesenchymal Stem Cells. Pharmaceutics. 2021;13:1448. doi: 10.3390/pharmaceutics13091448. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

