Anticancer and Antiphytopathogenic Activity of Fluorinated Isatins and Their Water-Soluble Hydrazone Derivatives
- PMID: 37894799
- PMCID: PMC10607100
- DOI: 10.3390/ijms242015119
Anticancer and Antiphytopathogenic Activity of Fluorinated Isatins and Their Water-Soluble Hydrazone Derivatives
Abstract
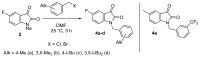
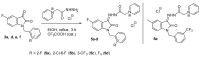
A series of new fluorinated 1-benzylisatins was synthesized in high yields via a simple one-pot procedure in order to explore the possible effect of ortho-fluoro (3a), chloro (3b), or bis-fluoro (3d) substitution on the biological activity of this pharmacophore. Furthermore, the new isatins could be converted into water-soluble isatin-3-hydrazones using their acid-catalyzed reaction with Girard's reagent P and its dimethyl analog. The cytotoxic action of these substances is associated with the induction of apoptosis caused by mitochondrial membrane dissipation and stimulated reactive oxygen species production in tumor cells. In addition, compounds 3a and 3b exhibit platelet antiaggregation activity at the level of acetylsalicylic acid, and the whole series of fluorine-containing isatins does not adversely affect the hemostasis system as a whole. Among the new water-soluble pyridinium isatin-3-acylhydrazones, compounds 7c and 5c,e exhibit the highest antagonistic effect against phytopathogens of bacterial and fungal origin and can be considered useful leads for combating plant diseases.
Keywords: antiphytopathogenes; cancer; crystal structure; cytotoxicity; hydrazones; isatin; quaternary ammonium compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Zhang S., Huang D., Wu J., Wang Z. Decade Advance of Isatin in Three-component Reactions. Asian J. Org. Chem. 2023;12:e202200591. doi: 10.1002/ajoc.202200591. - DOI
-
- Pradeep S.D., Mohanan P.V. Advancements in Schiff Bases of 1H-Indole-2,3-dione: A Versatile Heterocyclic Compound in Pharmacological Field. Mini-Rev. Org. Chem. 2023;20:45–54. doi: 10.2174/1570193X19666220309142035. - DOI
-
- Nath R., Pathania S., Grover G., Akhtar M. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020;1222:128900. doi: 10.1016/j.molstruc.2020.128900. - DOI
-
- Gupta A.K., Tulsyan S., Bharadwaj M., Mehrotra R. Systematic Review on Cytotoxic and Anticancer Potential of N-Substituted Isatins as Novel Class of Compounds Useful in Multidrug-Resistant Cancer Therapy: In Silico and In Vitro Analysis. Top. Curr. Chem. 2019;377:15. doi: 10.1007/s41061-019-0240-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

