Pharmacological Inhibition of Myostatin in a Mouse Model of Typical Nemaline Myopathy Increases Muscle Size and Force
- PMID: 37894805
- PMCID: PMC10606666
- DOI: 10.3390/ijms242015124
Pharmacological Inhibition of Myostatin in a Mouse Model of Typical Nemaline Myopathy Increases Muscle Size and Force
Abstract
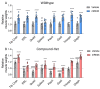
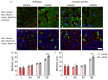
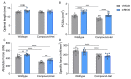
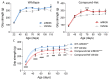
Nemaline myopathy is one of the most common non-dystrophic congenital myopathies. Individuals affected by this condition experience muscle weakness and muscle smallness, often requiring supportive measures like wheelchairs or respiratory support. A significant proportion of patients, approximately one-third, exhibit compound heterozygous nebulin mutations, which usually give rise to the typical form of the disease. Currently, there are no approved treatments available for nemaline myopathy. Our research explored the modulation of myostatin, a negative regulator of muscle mass, in combating the muscle smallness associated with the disease. To investigate the effect of myostatin inhibition, we employed a mouse model with compound heterozygous nebulin mutations that mimic the typical form of the disease. The mice were treated with mRK35, a myostatin antibody, through weekly intraperitoneal injections of 10 mg/kg mRK35, commencing at two weeks of age and continuing until the mice reached four months of age. The treatment resulted in an increase in body weight and an approximate 20% muscle weight gain across most skeletal muscles, without affecting the heart. The minimum Feret diameter of type IIA and IIB fibers exhibited an increase in compound heterozygous mice, while only type IIB fibers demonstrated an increase in wild-type mice. In vitro mechanical experiments conducted on intact extensor digitorum longus muscle revealed that mRK35 augmented the physiological cross-sectional area of muscle fibers and enhanced absolute tetanic force in both wild-type and compound heterozygous mice. Furthermore, mRK35 administration improved grip strength in treated mice. Collectively, these findings indicate that inhibiting myostatin can mitigate the muscle deficits in nebulin-based typical nemaline myopathy, potentially serving as a much-needed therapeutic option.
Keywords: myostatin; nebulin; nemaline myopathy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Muscle weakness in respiratory and peripheral skeletal muscles in a mouse model for nebulin-based nemaline myopathy.Neuromuscul Disord. 2017 Jan;27(1):83-89. doi: 10.1016/j.nmd.2016.10.004. Epub 2016 Oct 25. Neuromuscul Disord. 2017. PMID: 27890461 Free PMC article.
-
Myostatin inhibition using mRK35 produces skeletal muscle growth and tubular aggregate formation in wild type and TgACTA1D286G nemaline myopathy mice.Hum Mol Genet. 2018 Feb 15;27(4):638-648. doi: 10.1093/hmg/ddx431. Hum Mol Genet. 2018. PMID: 29293963 Free PMC article.
-
Deleting exon 55 from the nebulin gene induces severe muscle weakness in a mouse model for nemaline myopathy.Brain. 2013 Jun;136(Pt 6):1718-31. doi: 10.1093/brain/awt113. Epub 2013 May 28. Brain. 2013. PMID: 23715096 Free PMC article.
-
A childhood-onset nemaline myopathy caused by novel heterozygote variants in the nebulin gene with literature review.Acta Neurol Belg. 2020 Dec;120(6):1351-1360. doi: 10.1007/s13760-019-01230-3. Epub 2019 Nov 6. Acta Neurol Belg. 2020. PMID: 31696431 Review.
-
Recent advances in nemaline myopathy.Neuromuscul Disord. 2021 Oct;31(10):955-967. doi: 10.1016/j.nmd.2021.07.012. Epub 2021 Jul 24. Neuromuscul Disord. 2021. PMID: 34561123 Review.
Cited by
-
Inhibiting Myostatin Expression by the Antisense Oligonucleotides Improves Muscle Wasting in a Chronic Kidney Disease Mouse Model.Int J Mol Sci. 2025 Mar 27;26(7):3098. doi: 10.3390/ijms26073098. Int J Mol Sci. 2025. PMID: 40243849 Free PMC article.
-
Characterization of NEB pathogenic variants in patients reveals novel nemaline myopathy disease mechanisms and omecamtiv mecarbil force effects.Acta Neuropathol. 2024 Apr 18;147(1):72. doi: 10.1007/s00401-024-02726-w. Acta Neuropathol. 2024. PMID: 38634969 Free PMC article.
References
-
- Moreno C.A.M., Artilheiro M.C., Fonseca A., Camelo C.G., de Medeiros G.C., Sassi F.C., de Andrade C.R.F., Donkervoort S., Silva A.M.S., Dalfior-Junior L., et al. Clinical Manifestation of Nebulin-Associated Nemaline Myopathy. Neurol. Genet. 2023;9:e200056. doi: 10.1212/NXG.0000000000200056. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

