miR-632 Induces DNAJB6 Inhibition Stimulating Endothelial-to-Mesenchymal Transition and Fibrosis in Marfan Syndrome Aortopathy
- PMID: 37894814
- PMCID: PMC10607153
- DOI: 10.3390/ijms242015133
miR-632 Induces DNAJB6 Inhibition Stimulating Endothelial-to-Mesenchymal Transition and Fibrosis in Marfan Syndrome Aortopathy
Abstract
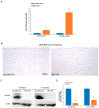
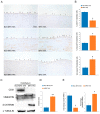
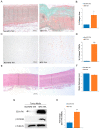
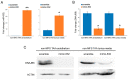
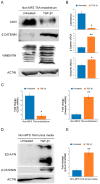
Marfan syndrome (MFS) is a connective tissue disorder caused by FBN1 gene mutations leading to TGF-β signaling hyperactivation, vascular wall weakness, and thoracic aortic aneurysms (TAAs). The pathogenetic mechanisms are not completely understood and patients undergo early vascular surgery to prevent TAA ruptures. We previously reported miR-632 upregulation in MFS TAA tissues compared with non-genetic TAA tissues. DNAJB6 is a gene target of miR-632 in cancer and plays a critical role in blocking epithelial-to-mesenchymal transition by inhibiting the Wnt/β catenin pathway. TGF-β signaling also activates Wnt/β catenin signaling and induces endothelial-to-mesenchymal transition (End-Mt) and fibrosis. We documented that miR-632 upregulation correlated with DNAJB6 expression in both the endothelium and the tunica media of MFS TAA (p < 0.01). Wnt/β catenin signaling, End-Mt, and fibrosis markers were also upregulated in MFS TAA tissues (p < 0.05, p < 0.01 and p < 0.001). Moreover, miR-632 overexpression inhibited DNAJB6, inducing Wnt/β catenin signaling, as well as End-Mt and fibrosis exacerbation (p < 0.05 and p < 0.01). TGF-β1 treatment also determined miR-632 upregulation (p < 0.01 and p < 0.001), with the consequent activation of the aforementioned processes. Our study provides new insights about the pathogenetic mechanisms in MFS aortopathy. Moreover, the high disease specificity of miR-632 and DNAJB6 suggests new potential prognostic factors and/or therapeutic targets in the progression of MFS aortopathy.
Keywords: DNAJB6; Marfan syndrome; TGF-β1; aortic wall degeneration; endothelial-to-mesenchymal transition; fibrosis; miR-632; thoracic aortic aneurysms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

