Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways
- PMID: 37894865
- PMCID: PMC10606873
- DOI: 10.3390/ijms242015186
Endoplasmic Reticulum Stress Promotes the Expression of TNF-α in THP-1 Cells by Mechanisms Involving ROS/CHOP/HIF-1α and MAPK/NF-κB Pathways
Abstract
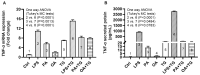
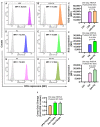
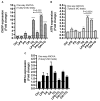
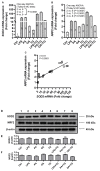
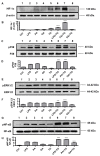
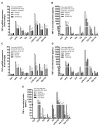
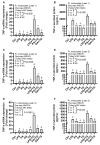
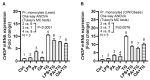
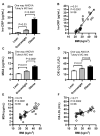
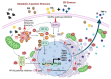
Obesity and metabolic syndrome involve chronic low-grade inflammation called metabolic inflammation as well as metabolic derangements from increased endotoxin and free fatty acids. It is debated whether the endoplasmic reticulum (ER) stress in monocytic cells can contribute to amplify metabolic inflammation; if so, by which mechanism(s). To test this, metabolic stress was induced in THP-1 cells and primary human monocytes by treatments with lipopolysaccharide (LPS), palmitic acid (PA), or oleic acid (OA), in the presence or absence of the ER stressor thapsigargin (TG). Gene expression of tumor necrosis factor (TNF)-α and markers of ER/oxidative stress were determined by qRT-PCR, TNF-α protein by ELISA, reactive oxygen species (ROS) by DCFH-DA assay, hypoxia-inducible factor 1-alpha (HIF-1α), p38, extracellular signal-regulated kinase (ERK)-1,2, and nuclear factor kappa B (NF-κB) phosphorylation by immunoblotting, and insulin sensitivity by glucose-uptake assay. Regarding clinical analyses, adipose TNF-α was assessed using qRT-PCR/IHC and plasma TNF-α, high-sensitivity C-reactive protein (hs-CRP), malondialdehyde (MDA), and oxidized low-density lipoprotein (OX-LDL) via ELISA. We found that the cooperative interaction between metabolic and ER stresses promoted TNF-α, ROS, CCAAT-enhancer-binding protein homologous protein (CHOP), activating transcription factor 6 (ATF6), superoxide dismutase 2 (SOD2), and nuclear factor erythroid 2-related factor 2 (NRF2) expression (p ≤ 0.0183),. However, glucose uptake was not impaired. TNF-α amplification was dependent on HIF-1α stabilization and p38 MAPK/p65 NF-κB phosphorylation, while the MAPK/NF-κB pathway inhibitors and antioxidants/ROS scavengers such as curcumin, allopurinol, and apocynin attenuated the TNF-α production (p ≤ 0.05). Individuals with obesity displayed increased adipose TNF-α gene/protein expression as well as elevated plasma levels of TNF-α, CRP, MDA, and OX-LDL (p ≤ 0.05). Our findings support a metabolic-ER stress cooperativity model, favoring inflammation by triggering TNF-α production via the ROS/CHOP/HIF-1α and MAPK/NF-κB dependent mechanisms. This study also highlights the therapeutic potential of antioxidants in inflammatory conditions involving metabolic/ER stresses.
Keywords: CHOP; ER stress; HIF-1α; MAPK/NF-κB; ROS; TNF-α; inflammation; metabolic stress; metabolic syndrome; obesity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

 or
or  ).
).References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

