The Role of the Oxidative State and Innate Immunity Mediated by TLR7 and TLR9 in Lupus Nephritis
- PMID: 37894915
- PMCID: PMC10607473
- DOI: 10.3390/ijms242015234
The Role of the Oxidative State and Innate Immunity Mediated by TLR7 and TLR9 in Lupus Nephritis
Abstract
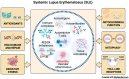
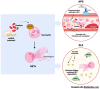
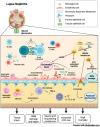
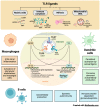
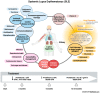
Lupus nephritis (LN) is a severe complication of systemic lupus erythematosus (SLE) and is considered one of the leading causes of mortality. Multiple immunological pathways are involved in the pathogenesis of SLE, which makes it imperative to deepen our knowledge about this disease's immune-pathological complexity and explore new therapeutic targets. Since an altered redox state contributes to immune system dysregulation, this document briefly addresses the roles of oxidative stress (OS), oxidative DNA damage, antioxidant enzymes, mitochondrial function, and mitophagy in SLE and LN. Although adaptive immunity's participation in the development of autoimmunity is undeniable, increasing data emphasize the importance of innate immunity elements, particularly the Toll-like receptors (TLRs) that recognize nucleic acid ligands, in inflammatory and autoimmune diseases. Here, we discuss the intriguing roles of TLR7 and TLR9 in developing SLE and LN. Also included are the essential characteristics of conventional treatments and some other novel and little-explored alternatives that offer options to improve renal function in LN.
Keywords: DNA damage; antioxidants; lupus nephritis; mitochondrial function; mitophagy; oxidative stress; systemic lupus erythematosus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

