Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis
- PMID: 37894973
- PMCID: PMC10607574
- DOI: 10.3390/ijms242015294
Effect of the Functional VP1 Unique Region of Human Parvovirus B19 in Causing Skin Fibrosis of Systemic Sclerosis
Abstract
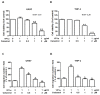
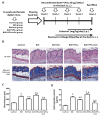
Human parvovirus B19 (B19V) is a single-stranded non-enveloped DNA virus of the family Parvoviridae that has been associated with various autoimmune disorders. Systemic sclerosis (SSc) is an autoimmune connective tissue disorder with high mortality and has been linked to B19V infection. However, the precise mechanism underlying the B19V contribution to the development of SSc remains uncertain. This study investigated the impacts of the functional B19V-VP1 unique region (VP1u) in macrophages and bleomycin (BLE)-induced SSc mice. Cell experimental data showed that significantly decreased viability and migration of both B19V-VP1u-treated U937 and THP-1 macrophages are detected in the presence of celastrol. Significantly increased MMP9 activity and elevated NF-kB, MMP9, IL-6, TNF-α, and IL-1β expressions were detected in both B19V-VP1u-treated U937 and THP-1 macrophages. Conversely, celastrol revealed an inhibitory effect on these molecules. Notably, celastrol intervened in this pathogenic process by suppressing the sPLA2 activity of B19V-VP1u and subsequently reducing the inflammatory response. Notably, the administration of B19V-VP1u exacerbated BLE-induced skin fibrosis in mice, with augmented expressions of TGF-β, IL-6, IL-17A, IL-18, and TNF-α, ultimately leading to α-SMA and collagen I deposits in the dermal regions of BLE-induced SSc mice. Altogether, this study sheds light on parvovirus B19 VP1u linked to scleroderma and aggravated dermal fibrosis.
Keywords: VP1 unique region (VP1u); fibrosis; human parvovirus B19 (B19); macrophages; systemic sclerosis (SSc).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Török T.J. Parvovirus B19 and human disease. Adv. Intern. Med. 1992;37:431–455. - PubMed
-
- Lombardo E., Ramírez J.C., Garcia J., Almendral J.M. Complementary roles of multiple nuclear targeting signals in the capsid proteins of the parvovirus minute virus of mice during assembly and onset of infection. J. Virol. 2002;76:7049–7059. doi: 10.1128/JVI.76.14.7049-7059.2002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

