Recent Advances in In Vivo Somatic Cell Gene Modification in Newborn Pups
- PMID: 37894981
- PMCID: PMC10607593
- DOI: 10.3390/ijms242015301
Recent Advances in In Vivo Somatic Cell Gene Modification in Newborn Pups
Abstract
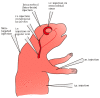
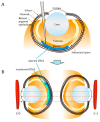
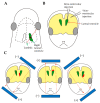
Germline manipulation at the zygote stage using the CRISPR/Cas9 system has been extensively employed for creating genetically modified animals and maintaining established lines. However, this approach requires a long and laborious task. Recently, many researchers have attempted to overcome these limitations by generating somatic mutations in the adult stage through tail vein injection or local administration of CRISPR reagents, as a new strategy called "in vivo somatic cell genome editing". This approach does not require manipulation of early embryos or strain maintenance, and it can test the results of genome editing in a short period. The newborn is an ideal stage to perform in vivo somatic cell genome editing because it is immune-privileged, easily accessible, and only a small amount of CRISPR reagents is required to achieve somatic cell genome editing throughout the entire body, owing to its small size. In this review, we summarize in vivo genome engineering strategies that have been successfully demonstrated in newborns. We also report successful in vivo genome editing through the neonatal introduction of genome editing reagents into various sites in newborns (as exemplified by intravenous injection via the facial vein), which will be helpful for creating models for genetic diseases or treating many genetic diseases.
Keywords: CRISPR/Cas9; genetically modified animal; genome editing; in vivo genome engineering; newborn; somatic mutation.
Conflict of interest statement
The founding sponsors had no role in the study design; the data collection, analyses, or interpretation; the writing of the manuscript; or the decision to publish the results.
Figures
References
-
- National Academies of Sciences, Engineering, and Medicine. National Academy of Medicine. National Academy of Sciences. Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations . Human Genome Editing: Science, Ethics, and Governance. National Academies Press; Washington, DC, USA: 2017. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

