Polyamines in Ovarian Aging and Disease
- PMID: 37895010
- PMCID: PMC10607840
- DOI: 10.3390/ijms242015330
Polyamines in Ovarian Aging and Disease
Abstract
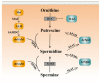
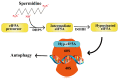
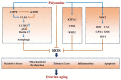
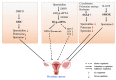
Ovarian aging and disease-related decline in fertility are challenging medical and economic issues with an increasing prevalence. Polyamines are a class of polycationic alkylamines widely distributed in mammals. They are small molecules essential for cell growth and development. Polyamines alleviate ovarian aging through various biological processes, including reproductive hormone synthesis, cell metabolism, programmed cell death, etc. However, an abnormal increase in polyamine levels can lead to ovarian damage and promote the development of ovarian disease. Therefore, polyamines have long been considered potential therapeutic targets for aging and disease, but their regulatory roles in the ovary deserve further investigation. This review discusses the mechanisms by which polyamines ameliorate human ovarian aging and disease through different biological processes, such as autophagy and oxidative stress, to develop safe and effective polyamine targeted therapy strategies for ovarian aging and the diseases.
Keywords: aging; autophagy; cancer; disease; ovary; polyamine.
Conflict of interest statement
The authors declared no conflict of interest.
Figures
References
-
- Vrijsen S., Besora-Casals L., van Veen S., Zielich J., Van den Haute C., Hamouda N.N., Fischer C., Ghesquière B., Tournev I., Agostinis P., et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc. Natl. Acad. Sci. USA. 2020;117:31198–31207. doi: 10.1073/pnas.1922342117. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

