Vaping-Induced Proteolysis Causes Airway Surface Dehydration
- PMID: 37895029
- PMCID: PMC10607227
- DOI: 10.3390/ijms242015348
Vaping-Induced Proteolysis Causes Airway Surface Dehydration
Abstract
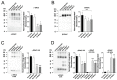
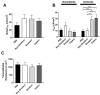
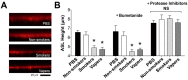
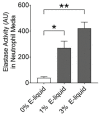
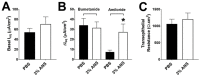
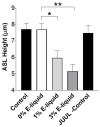
Proteases such as neutrophil elastase cleave and activate the epithelial sodium channel (ENaC), causing airway dehydration. Our current study explores the impact of increased protease levels in vapers' airways on ENaC activity and airway dehydration. Human bronchial epithelial cultures (HBECs) were exposed to bronchoalveolar lavage fluid (BALF) from non-smokers, smokers and vapers. Airway surface liquid (ASL) height was measured by confocal microscopy as a marker of hydration. ENaC cleavage was measured by Western blotting. Human peripheral blood neutrophils were treated with a menthol-flavored e-liquid (Juul), and the resulting secretions were added to HBECs. BALF from smokers and vapers significantly and equally increased ENaC activity and decreased ASL height. The ASL height decrease was attenuated by protease inhibitors. Non-smokers' BALF had no effect on ENaC or ASL height. BALF from smokers and vapers, but not non-smokers, induced ENaC cleavage. E-liquid-treated neutrophil secretions cleaved ENaC and decreased ASL height. Our study demonstrated that elevated protease levels in vapers' airways have functional significance since they can activate ENaC, resulting in airway dehydration. Lung dehydration contributes to diseases like cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and asthma. Thus, our data predict that vaping, like smoking, will cause airway surface dehydration that likely leads to lung disease.
Keywords: ENaC; airway surface liquid; bronchoalveolar lavage fluid; e-cigarettes; elastase; menthol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Talih S., Salman R., El-Hage R., Karam E., Salam S., Karaoghlanian N., El-Hellani A., Saliba N., Shihadeh A. A comparison of the electrical characteristics, liquid composition, and toxicant emissions of JUUL USA and JUUL UK e-cigarettes. Sci. Rep. 2020;10:7322. doi: 10.1038/s41598-020-64414-5. - DOI - PMC - PubMed
-
- Pulvers K., Rice M., Ahluwalia J.S., Arnold M.J., Marez C., Nollen N.L. “It is the One Thing that has Worked”: Facilitators and barriers to switching to nicotine salt pod system e-cigarettes among African American and Latinx people who smoke: A content analysis. Harm Reduct. J. 2021;18:98. doi: 10.1186/s12954-021-00543-y. - DOI - PMC - PubMed
-
- Leone F.T., Carlsen K.H., Chooljian D., Crotty Alexander L.E., Detterbeck F.C., Eakin M.N., Evers-Casey S., Farber H.J., Folan P., Kathuria H., et al. Recommendations for the Appropriate Structure, Communication, and Investigation of Tobacco Harm Reduction Claims. An Official American Thoracic Society Policy Statement. Am. J. Respir. Crit. Care Med. 2018;198:e90–e105. doi: 10.1164/rccm.201808-1443ST. - DOI - PMC - PubMed
-
- Park-Lee E., Ren C., Sawdey M.D., Gentzke A.S., Cornelius M., Jamal A., Cullen K.A. Notes from the Field: E-Cigarette Use Among Middle and High School Students—National Youth Tobacco Survey, United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1387–1389. doi: 10.15585/mmwr.mm7039a4. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

