Ionic Mechanisms of Propagated Repolarization in a One-Dimensional Strand of Human Ventricular Myocyte Model
- PMID: 37895058
- PMCID: PMC10607672
- DOI: 10.3390/ijms242015378
Ionic Mechanisms of Propagated Repolarization in a One-Dimensional Strand of Human Ventricular Myocyte Model
Abstract
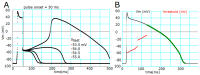
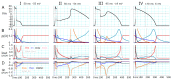
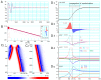
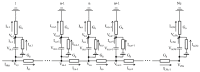
Although repolarization has been suggested to propagate in cardiac tissue both theoretically and experimentally, it has been challenging to estimate how and to what extent the propagation of repolarization contributes to relaxation because repolarization only occurs in the course of membrane excitation in normal hearts. We established a mathematical model of a 1D strand of 600 myocytes stabilized at an equilibrium potential near the plateau potential level by introducing a sustained component of the late sodium current (INaL). By applying a hyperpolarizing stimulus to a small part of the strand, we succeeded in inducing repolarization which propagated along the strand at a velocity of 1~2 cm/s. The ionic mechanisms responsible for repolarization at the myocyte level, i.e., the deactivation of both the INaL and the L-type calcium current (ICaL), and the activation of the rapid component of delayed rectifier potassium current (IKr) and the inward rectifier potassium channel (IK1), were found to be important for the propagation of repolarization in the myocyte strand. Using an analogy with progressive activation of the sodium current (INa) in the propagation of excitation, regenerative activation of the predominant magnitude of IK1 makes the myocytes at the wave front start repolarization in succession through the electrical coupling via gap junction channels.
Keywords: early afterdepolarization; human ventricular myocyte; late sodium current; mathematical 1D strand model; repolarization propagation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

