Comparative Benchmarking of Optical Genome Mapping and Chromosomal Microarray Reveals High Technological Concordance in CNV Identification and Additional Structural Variant Refinement
- PMID: 37895217
- PMCID: PMC10667989
- DOI: 10.3390/genes14101868
Comparative Benchmarking of Optical Genome Mapping and Chromosomal Microarray Reveals High Technological Concordance in CNV Identification and Additional Structural Variant Refinement
Abstract
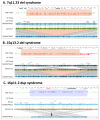
The recommended practice for individuals suspected of a genetic etiology for disorders including unexplained developmental delay/intellectual disability (DD/ID), autism spectrum disorders (ASD), and multiple congenital anomalies (MCA) involves a genetic testing workflow including chromosomal microarray (CMA), Fragile-X testing, karyotype analysis, and/or sequencing-based gene panels. Since genomic imbalances are often found to be causative, CMA is recommended as first tier testing for many indications. Optical genome mapping (OGM) is an emerging next generation cytogenomic technique that can detect not only copy number variants (CNVs), triploidy and absence of heterozygosity (AOH) like CMA, but can also define the location of duplications, and detect other structural variants (SVs), including balanced rearrangements and repeat expansions/contractions. This study compares OGM to CMA for clinically reported genomic variants, some of these samples also have structural characterization by fluorescence in situ hybridization (FISH). OGM was performed on IRB approved, de-identified specimens from 55 individuals with genomic abnormalities previously identified by CMA (61 clinically reported abnormalities). SVs identified by OGM were filtered by a control database to remove polymorphic variants and against an established gene list to prioritize clinically relevant findings before comparing with CMA and FISH results. OGM results showed 100% concordance with CMA findings for pathogenic variants and 98% concordant for all pathogenic/likely pathogenic/variants of uncertain significance (VUS), while also providing additional insight into the genomic structure of abnormalities that CMA was unable to provide. OGM demonstrates equivalent performance to CMA for CNV and AOH detection, enhanced by its ability to determine the structure of the genome. This work adds to an increasing body of evidence on the analytical validity and ability to detect clinically relevant abnormalities identified by CMA. Moreover, OGM identifies translocations, structures of duplications and complex CNVs intractable by CMA, yielding additional clinical utility.
Keywords: AOH; CMA; CNVs; OGM; SVs; Saphyr; absence of heterozygosity; aneuploidy; chromosomal microarray; optical genome mapping; triploidy.
Conflict of interest statement
A.W.C.P., B.C., A.C. and A.R.H. are employees at Bionano Genomics Inc. and own stock shares and options of Bionano Genomics Inc. M.S. is an employee at Bionano Laboratories and owns stock shares and options of Bionano Genomics Inc. H.B. is a former employee and owns Bionano stock shares and options.
Figures



References
-
- Online Mendelian Inheritance in Man, OMIM® McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD) 2020. [(accessed on 1 August 2022)]. Available online: https://omim.org/
-
- Miller D.T., Adam M.P., Aradhya S., Biesecker L.G., Brothman A.R., Carter N.P., Church D.M., Crolla J.A., Eichler E.E., Epstein C.J., et al. Consensus Statement: Chromosomal Microarray Is a First-Tier Clinical Diagnostic Test for Individuals with Developmental Disabilities or Congenital Anomalies. Am. J. Hum. Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. - DOI - PMC - PubMed
-
- Shevell M., Ashwal S., Donley D., Flint J., Gingold M., Hirtz D., Majnemer A., Noetzel M., Sheth R.D. Practice Parameter: Evaluation of the Child with Global Developmental Delay [RETIRED]: Report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology. 2003;60:367–380. doi: 10.1212/01.WNL.0000031431.81555.16. - DOI - PubMed
-
- Moeschler J.B., Shevell M., Committee on Genetics. Moeschler J.B., Shevell M., Saul R.A., Chen E., Freedenberg D.L., Hamid R., Jones M.C., et al. Comprehensive Evaluation of the Child with Intellectual Disability or Global Developmental Delays. Pediatrics. 2014;134:e903–e918. doi: 10.1542/peds.2014-1839. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

