Prostate Cancer Microvascular Routes: Exploration and Measurement Strategies
- PMID: 37895416
- PMCID: PMC10608780
- DOI: 10.3390/life13102034
Prostate Cancer Microvascular Routes: Exploration and Measurement Strategies
Abstract
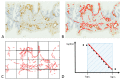
Angiogenesis is acknowledged as a pivotal feature in the pathology of human cancer. Despite the absence of universally accepted markers for gauging the comprehensive angiogenic activity in prostate cancer (PCa) that could steer the formulation of focused anti-angiogenic treatments, the scrutiny of diverse facets of tumoral blood vessel development may furnish significant understanding of angiogenic processes. Malignant neoplasms, encompassing PCa, deploy a myriad of strategies to secure an adequate blood supply. These modalities range from sprouting angiogenesis and vasculogenesis to intussusceptive angiogenesis, vascular co-option, the formation of mosaic vessels, vasculogenic mimicry, the conversion of cancer stem-like cells into tumor endothelial cells, and vascular pruning. Here we provide a thorough review of these angiogenic mechanisms as they relate to PCa, discuss their prospective relevance for predictive and prognostic evaluations, and outline the prevailing obstacles in quantitatively evaluating neovascularization via histopathological examinations.
Keywords: biomarkers; fractals; microvessel density; prostate cancer; vasculature.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

