The Impact of Heating Rate on the Kinetics of the Nitriding Process for 52100 Steel
- PMID: 37895690
- PMCID: PMC10608022
- DOI: 10.3390/ma16206708
The Impact of Heating Rate on the Kinetics of the Nitriding Process for 52100 Steel
Abstract
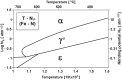
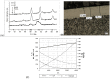
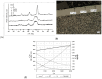
The aim of this study was to determine the impact of the heating rate of steel balls made of AISI 52100 alloy steel on the kinetics and efficiency of the gas nitriding process when carried out using a chemical reactor with precise thermo-gravimetric measurements, which allowed for changes in sample mass during heating and nitriding to be monitored with an accuracy of 50 µg. In the chemical reactor, the examined alloy steel was subjected to a heating process at the selected nitriding temperature of 590 °C. Two heating variants were used: the first variant relied on heating to the nitriding temperature with different rates-1 °C per minute, 2 °C per minute, 5 °C per minute and 10 °C per minute, respectively-whereas the second variant relied on the fast-25 °C per minute-heating of treated specimens to a temperature of 475 °C, at which, the nitrogenous potential of the atmosphere promotes faster nitrogen diffusion deep into the nitrided substrate, followed by reheating up to the nitriding temperature at different rates: 1 °C per minute, 2 °C per minute, 5 °C per minute, and 10 °C per minute, respectively. To evaluate the impact of heating rate kinetics and effectiveness during nitriding on the obtained surface layer quality, we investigated the phase composition, microhardness distribution, and thickness of the obtained diffusion layers. It was found that heating to a temperature of 475 °C in the nitriding process does not significantly affect the average mass gain of a sample. Above this temperature, within the range of nitriding temperatures, the extension of time increases the sample's mass gain. Simultaneously, it was found that the use of a constant heating rate allows for thicker nitrided layers and a greater sample hardness to be obtained. Dual-stage heating, in turn, is more effective in the context of sample mass gain per time unit.
Keywords: gas nitriding; kinetics and efficiency of the process; nitride phases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Frączek T. Niekonwencjonalne Niskotemperaturowe Azotowanie Jarzeniowe Materiałów Metalicznych. Wydawnictwo WIPMiFS Politechniki Czestochowskiej; Częstochowa, Poland: 2011.
-
- Wang J., Zhang G., Sun J., Bao Y., Zhuang L., Wen H. Low Temperature Nitriding of Medium Carbon Steel. Vacuum. 2006;80:855–859. doi: 10.1016/j.vacuum.2005.10.008. - DOI
-
- Mittemeijer E.J. Steel Heat Treating Fundamentals and Processes. ASM International; Almere, The Netherlands: 2013. Fundamentals of Nitriding and Nitrocarburizing; pp. 619–646.
-
- Shen H., Wang L. Mechanism and Properties of Plasma Nitriding AISI 420 Stainless Steel at Low Temperature and Anodic (Ground) Potential. Surf. Coat. Technol. 2020;403:126390. doi: 10.1016/j.surfcoat.2020.126390. - DOI
-
- Xie W., Chen Y., Chen D., Yang Y., Zhang C., Cui G., Wang Y. Low-Pressure Gas Nitriding of AISI 304 Austenitic Stainless Steel: Reducing the Precipitation of Chromium Nitrides. Mater. Res. Express. 2020;7:066406. doi: 10.1088/2053-1591/ab9bef. - DOI
LinkOut - more resources
Full Text Sources

