Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer's Disease in Rats
- PMID: 37895841
- PMCID: PMC10609711
- DOI: 10.3390/ph16101370
Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer's Disease in Rats
Abstract
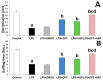
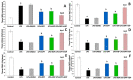
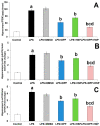
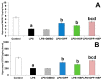
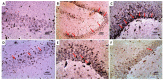
Alzheimer's disease (AD) is the most common form of neurodegenerative disorders worldwide. Its pathologic features include massive neuroinflammation with abnormal deposition of β-amyloid peptide in the cerebral tissues leading to degeneration of the brain neurons. Adverse effects associated with the traditional drugs used for the treatment of this pathological condition have directed the research efforts towards searching for alternative effective agents with minimal adverse effects. The aim of this study was to elucidate the potential ameliorative effects of dapagliflozin and/or hesperidin on Alzheimer's disease (AD) induced by lipopolysaccharide (LPS) injection in rats. In a rodent model of AD, the effect of dapagliflozin with or without hesperidin on the biochemical parameters and the behavioral tests as well as the histopathological parameters was determined. Each of dapagliflozin and hesperidin restored the behavioral tests to the reference values, augmented the antioxidant defense mechanisms, ameliorated the neuronal inflammatory responses, combatted the changes in Toll-like receptor-4 (TLR-4)/High-mobility group box 1 (HMGB1) protein signaling and receptors of advanced glycation end products (RAGE) levels, and restored the balance between the apoptotic signals and autophagy in the hippocampal tissues. Additionally, both agents exhibited an outstanding ability to combat LPS-induced perturbations in the histopathological and electron microscopic image of the brain tissues. These favorable effects were significantly encountered in the group treated with dapagliflozin/hesperidin combination when compared versus animals treated with either dapagliflozin or hesperidin. In conclusion, inhibition of the hippocampal HMGB1/TLR4/RAGE signaling, the pro-inflammatory axis, and apoptosis alongside augmentation of the antioxidant defenses and autophagy can be regarded as beneficial effects by which dapagliflozin/hesperidin combination may combat LPS-triggered AD.
Keywords: Alzheimer’s disease; apoptosis; autophagy; dapagliflozin; hesperidin; inflammatory cascade; lipopolysaccharide; oxidative stress; rats.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
Grants and funding
LinkOut - more resources
Full Text Sources

